Little Emily Borg died aged 5 after battling neuroblastoma. But it wasn’t the disease that killed her, as her family make a desperate plea to the government to prevent trauma for other sufferers
Oncology patient Emily Borg was cancer-free when she died at age five.
Her immune system had been destroyed by the treatment meant to save her life, so when she contracted a rare fungal infection weeks later, her body couldn’t fight it.
The best available antifungal drug was not approved for use in children, and even if Emily’s doctor requested its use on compassionate grounds, it would likely be denied.
By August 2023, the infection had reached Emily’s brain, causing six aneurysms and an ultimately fatal stroke.
Emily Borg, 5, died from a rare fungal infection after her immune system was compromised by treatment for liver cancer
“In the end it wasn’t the cancer that took her life, it was the treatment,” Emily’s aunt Kate Cantrell told AAP.
“All anyone could say was, ‘Emily was just very unlucky.'”
Dr. Cantrell wants to ensure this never happens to an Australian family again and is calling on the government to further invest in safer, kinder and more effective treatments for children with cancer.
When she was four and a half years old, Emily was diagnosed with the rare and extremely aggressive form of childhood cancer: neuroblastoma.
Doctors were forced to throw the sink full of treatments at her.
One of the medications Emily was given, thiotepa, was so toxic that her father had to bathe her every four hours.
Fortunately, her body responded perfectly.
She was declared to have no signs of illness, but due to the high risk of relapse, Emily had to undergo a bone marrow transplant that would destroy her immune system.
The medical team assured her loved ones that Emily was in the best possible position.
Since then, her family has wondered if they made the right decisions, but they soon learned that Australian children had relatively little choice.
Australia’s treatment protocol for neuroblastoma has seen just one change in the past decade, says Lucy Jones, CEO of Neuroblastoma Australia, meaning patients have not been able to replace the more toxic drugs with newer and safer alternatives.
Treatments for childhood diseases are also not as commercially viable, so Australian pharmaceutical companies tend to focus on common cancers in adults.
However, in the US and Europe, pharmaceutical companies receive government funding for pediatric cancer research, giving these regions greater access to innovative and leading solutions that leave less significant aftereffects.
Red tape can also block access to life-saving drugs, which must undergo clinical trials in Australia even if proven effective in the US and Europe, with the entire approval process taking an average of 18 months.
Even then, many will not be approved for children.
“All it does is delay access to that medicine and cost lives while people wait,” Ms Jones said.
As a result, families sometimes pay hundreds of thousands of dollars to send their children to hospitals abroad.
From the moment Emily was diagnosed, her family began seeking half a million dollars so she could travel to New York for a vaccine.
“Australian families are going bankrupt and burning out,” Dr Cantrell said.
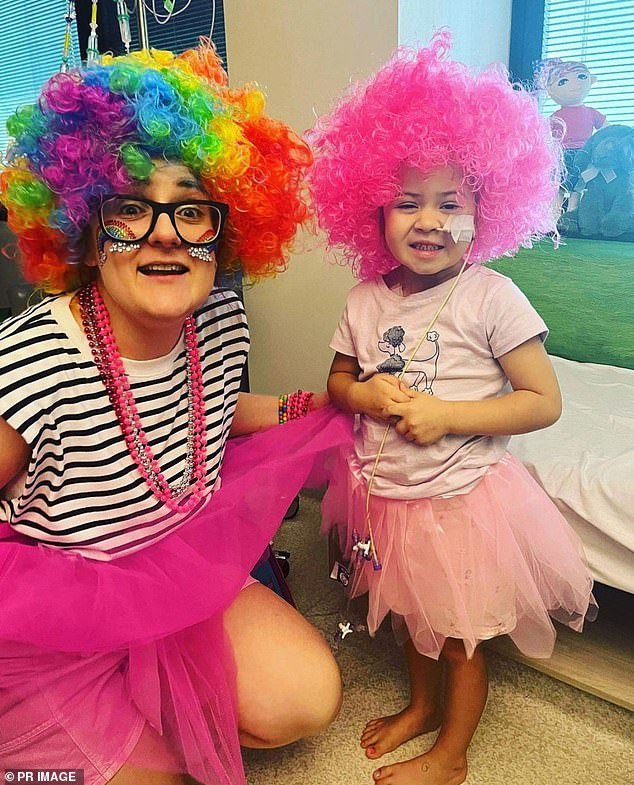
Emily and her aunt Kate Cantrell pose for a photo as her family calls for a cut in red tape for a drug that could have saved the five-year-old’s life
‘Until Emily was diagnosed, I never thought a child would be disadvantaged for living in Australia, the so-called lucky country.’
The government has provided $60 million to fund Zero Childhood Cancer, a program that offers genomic cancer testing to all children with the disease.
It has also offered grants for pediatric brain cancer research, committing $20 million in February to research into diffuse intrinsic pontine glioma (DIPG), and $15 million to research drug development for prostate and childhood cancers such as neuroblastoma.
While Ms Jones welcomed the funding commitments, to have a significant impact, each form of childhood cancer would need around $50 million a year due to the costs of clinical trials.
But if the government were to create a $100 million research pool for childhood cancer and ensure a concerted approach, it could tackle a variety of diseases and the consequences of common treatments, she said.
Dr. Cantrell paid tribute to Emily at Parliament House at the end of January when she chaired a Senate committee.
Emily called the tumor “the monster in her stomach” and once ran away when she heard a knock on the door, afraid that the ghost had returned.
Not knowing how to write yet, she texted her aunt with emojis, liberally using the symbols for poop and vomit.
Emily also fooled oncology staff, filling her urine sample cups with apple juice and hiding toy tubes in hospital drawers.
“She was a little firecracker,” Dr. Cantrell said.
‘I miss her energy, the lightness and the laughter she brings, I miss the sound of her voice.
“All childhood cancers are rare, but it’s not rare if it happens to you.”
Lifeline 13 11 14
Children’s helpline 1800 55 1800 (for people aged 5 to 25)
