What is anastrozole? What are the side effects? And am I eligible for the breast cancer drug? All your questions answered
Women at higher risk of developing breast cancer are offered a pill that halves their risk of developing the disease.
NHS England and British drug watchdogs have announced that the pill anastrozole will now be offered as a cancer preventative thanks to a new approval process that repurposes old drugs for new uses.
The health service’s chief executive, Amanda Pritchard, said: ‘It’s fantastic that this vital risk-reducing option can now help thousands of women and their families avoid the pain of a breast cancer diagnosis.’
Women already taking the drug have described it as a “gift” that allows them to live their lives with less worry about a possible cancer diagnosis.
But what exactly is anastrozole? Who is eligible? And are there any side effects?
Here MailOnline answers all your questions…
Traditionally, anastrozole has been used as a treatment for women suffering from breast cancer. But studies have shown that hormone therapy can also cut a woman’s chances of developing it in half
What is anastrozole and how does it work?
Anastrozole is a hormone therapy drug that has been used for years in the treatment of breast cancer.
It works by reducing the amount of aromatase the body can produce. After women go through menopause, this enzyme is essential for estrogen production.
This reduces the risk of breast cancer, because estrogen stimulates the growth of some versions of the disease.
What happened?
Although anastrozole has been used for years as a breast cancer drug, doctors can now easily prescribe it as a cancer preventative.
The preventive benefits of the drug have been known since 2017.
However, hurdles had to be overcome before it could be offered for this use.
After AstraZeneca’s patent on anastrozole expired, it became a generic drug, meaning it could be made by any drug manufacturer — a process that usually makes drugs cheaper.
However, it also offers companies little incentive to go through the steps of getting a generic drug approved for another medical use – in this case, cancer prevention rather than treatment.
However, the UK Medicines Repurpose Programme, set up in 2021 by the NHS, the government and the UK medicines and treatment watchdogs, is taking on this process.
A small number of women were previously prescribed the drug as a cancer prevention drug ‘off-label’, meaning doctors prescribed it outside of its officially approved use.
But the new official approval is expected to make it easier for more women to use it, with NHS chiefs saying around 289,000 women will be eligible.
How effective is it to prevent cancer?
According to clinical studies, breast cancer rates decreased by 49 percent among women at high risk of developing breast cancer who took anastrozole.
The study followed 4,000 postmenopausal women for 10 years and found that the reduced risk of breast cancer persisted even when women stopped taking the drug.
Breast cancer is most often diagnosed in women over the age of 50 who have gone through menopause; this accounts for 80 percent of all cases in Britain.
How often should patients take it?
Anastrozole is taken as a daily tablet.
Women prescribed the drug for breast cancer prevention will take it for five years between the ages of 50 and 69.
Who is eligible for the medication?
The NHS says around 289,000 women aged 50 to 69 with a moderate to high risk of breast cancer could be eligible for the drug.
Women are classified as at moderate risk if they have one close relative, such as a mother, sister or daughter, who has breast cancer.
Their risk of developing the disease is about one in six, compared to one in seven among the general population.
Women are considered high risk as two close relatives or one close relative and a second more distant relative – such as a grandmother or aunt – have breast cancer.
Their chance of developing the disease is one in three.
It is expected that women will be offered anastrozole after visiting their GP, who will look at their family history. However, this process may involve further consultation with a specialist.
What are the side effects?
The most common side effects are menopause-like symptoms.
More than one percent of women who take the drug experience hot flashes, sleep problems, fatigue and bad mood, as well as a dry or itchy vagina, mild pain and thinning or falling hair.
However, these side effects usually improve within the first few months of taking anastrozole.
Serious side effects occur in fewer than one in 100 women who use the medicine. These include painful or swollen muscles and joints, liver problems and blurred vision.
How many lives could it save?
No specific estimates exist for this.
But the NHS says that even if just a quarter of eligible women take up the offer of the drug, it will prevent 2,000 cases of breast cancer.
While not all of this will necessarily be fatal, it will spare these women from treatments such as surgery or grueling chemotherapy.
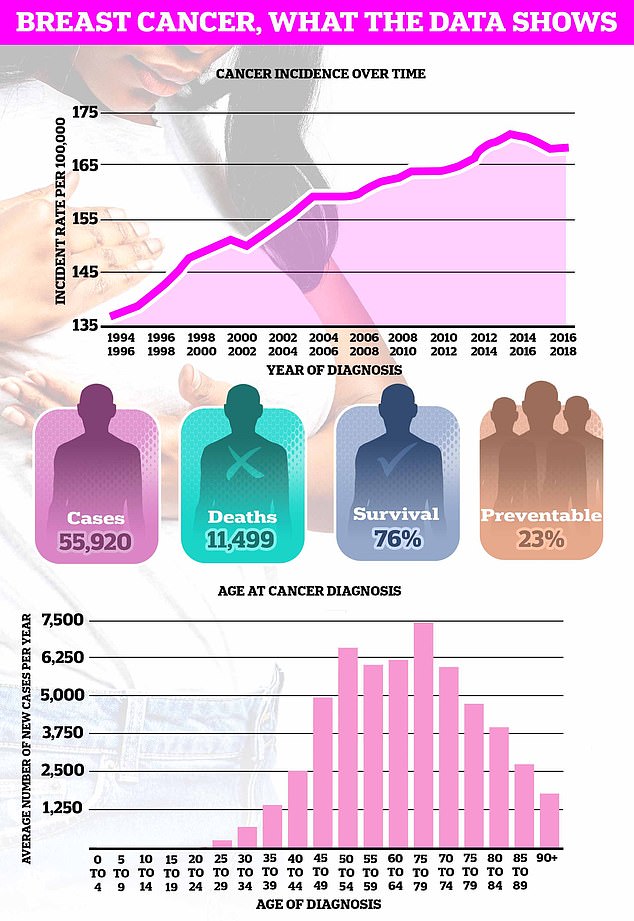
Breast cancer kills around 11,500 Britons a year, making it the fourth biggest cancer killer in Britain.
For women specifically, breast cancer is the second leading cause of death of all versions of the disease.
Why does the NHS think that not all eligible women will take the medication?
This is probably due to the side effects.
Some women will be more sensitive to it than others, or suffer more severe symptoms while taking the drug, so they may choose to stop taking it.
Because anastrozole is a cancer preventative, a woman taking the drug will have no evidence that it has stopped them from developing breast cancer.
Considering that even a woman at high risk for developing breast cancer has a greater chance of dodging the disease than of receiving a cancer diagnosis, some women may decide that taking the medication for five years is not worth it is.
How much does the medicine cost?
Anastrozole costs just 4p per tablet, meaning a five-year course costs a total of £70.
It would cost £5.3 million if 72,250 women took the medication for five years.
