Urgent recall of lifesaving medication amid warnings of ‘deadly’ consequences
Pharmaceutical giant Astellas Pharma has issued an urgent recall for two of its crucial drugs: Prograf and Astagraf XL – which could have fatal consequences for patients recovering from life-saving organ transplants.
Both medications are immunosuppressants prescribed to help transplant recipients avoid organ rejection, but shockingly, some capsules may be completely empty.
The ineffective pills would leave patients unprotected and at risk of developing life-threatening complications such as organ rejection.
The discovery of empty capsules raises alarming questions about manufacturing oversight and quality control, especially for drugs where even one missed dose can have catastrophic consequences.
The recall focuses on Prograf 0.5 mg capsules (lot no. 0E3353D) and Astagraf XL 0.5 mg capsules (lot no. 0R3092A), both of which have an expiration date of March 2026.
Prograf is used after heart, liver, kidney or lung transplants, while Astagraf XL is specifically prescribed for kidney transplants.
“In the case of life-sustaining organ transplants such as a heart transplant,” Astellas acknowledged in its recall, “the consequences of rejection initiated by ingestion of empty capsules can be fatal if the transplant fails.”
For transplant recipients, the drugs are the cornerstone of their survival.
Pharmaceutical giant Astellas Pharma has issued an urgent recall for two of its crucial medicines: Prograf and Astagraf XL – which may have been shipped with empty capsules
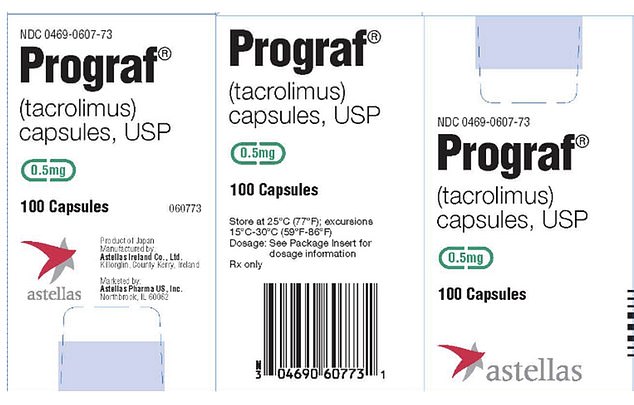
Prograf from Astellas Pharma is prescribed after heart, liver, kidney or lung transplants

Astellas’ Astagraf XL is usually prescribed after kidney transplants
Immunosuppressive medications such as Prograf and Astagraf XL prevent the body from rejecting transplanted organs, tissues, or cells.
A missed dose – or in this case, unknowingly taking an ineffective empty capsule – can disrupt this delicate balance and trigger an immune response that can lead to organ failure.
The stakes are particularly high for heart transplant patients, because there is no long-term replacement treatment, such as dialysis, for kidney failure.
Astellas Pharma, a global leader in the development of transplant medicines, has long been a trusted name in the pharmaceutical world.
Not all Prograf and Astagraf XL products are affected, but the recall includes the following:
- Prograf 0.5 mg capsules: lot no. 0E3353D, bottles of 100 pieces
- Astagraf XL 0.5 mg capsules: lot no. 0R3092A, bottles of 30 pieces

Astellas Pharma, a global leader in the development of transplant medicines, has long been a trusted name in the pharmaceutical world. The photo shows the headquarters in Tokyo, Japan
Patients are urged to immediately review their medications and consult their prescribers if they suspect they have a recalled product.
Wholesalers and pharmacies stocking these products have been instructed to contact Astellas directly for advice at 877-575-3437.
They must then report the incident to the The FDA’s MedWatch Program at 800-332-1088.
For patients who experience potential symptoms of organ rejection – such as fever, pain near the transplanted organ, or sudden changes in health – it is crucial to seek immediate medical attention.
