The new Ozempic? California drug manufacturer develops ‘remarkable’ drug MariTide that is just as effective and does not have to last forever
Early studies have shown that a monthly injectable weight loss drug may help patients shed more pounds than the blockbuster shot Ozempic.
Californian pharmaceutical company Amgen discovered that their new, experimental drug MariTide helped patients lose up to 14.5 percent of their body weight in just 12 weeks
This is compared to the weight loss of 15 and 21 percent with Ozempic and similar drugs such as Wegovy and Zepbound over a year.
Additionally, some study participants maintained weight loss for nearly five months after stopping the drug.
Ozempic sees that about two-thirds of patients regain all their lost weight a year after quitting.
Amgen says this means MariTide can be taken in lower doses, reducing the risk of weight loss.
The drug works in a slightly different way than the weight loss injections currently on the market, although it contains the same hunger hormone, GLP-1.
One part of the drug blocks the hormone GIP, which is believed to promote fat storage, while the other part mimics the activity of the GLP-1 hormones linked to feelings of fullness.
Amgen’s monthly injection is still a long way from reaching pharmacies. The latest data is part of a preliminary data set from a Phase 1 study involving just 49 subjects.
However, a phase 2 trial is underway, involving more participants.
Unlike Wegovy, Ozempic and ZepBound, Amgen’s injectable solution would be given monthly. This would appeal to patients dissatisfied with the standard weekly injection regimen, and could translate into lower costs
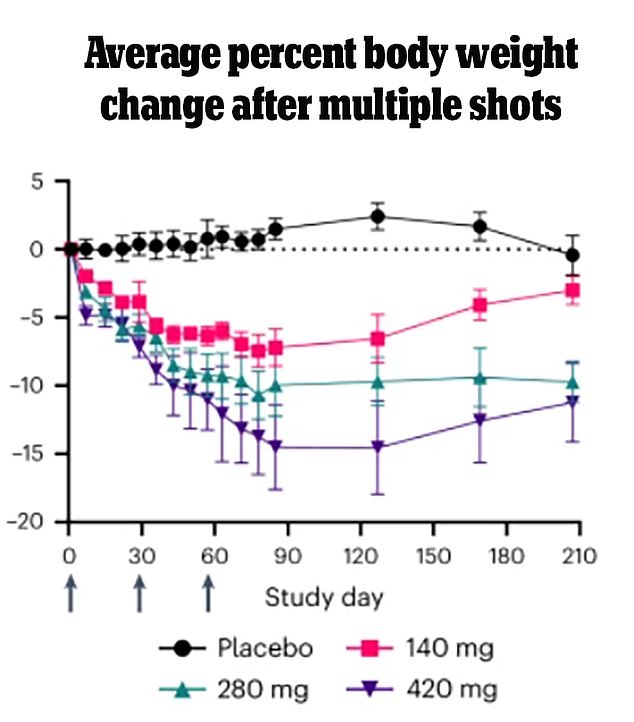
People taking MariTide lost up to 14.5 percent of their total body weight in just three months. They were also able to maintain the weight loss after stopping the last dose
The findings are notable because the current weight loss shots on the market only seem to work as long as a person takes them, and regains the weight when they stop.
The latest results published in the journal Nature metabolism have been hotly anticipated since company leadership shared initial clinical trial results at a 2022 conference.
In the study, 49 patients were given different doses of the drug, ranging from 21 milligrams to 840 milligrams.
These patients were obese, but had no other associated health problems, such as diabetes.
Eight patients who received the highest dosage saw an average weight loss of 14.5 percent over a period of about three months.
Data from the study shows that weight loss continued even after patients stopped taking the medication. Participants maintained their maximum weight loss until about two months after their last dose.
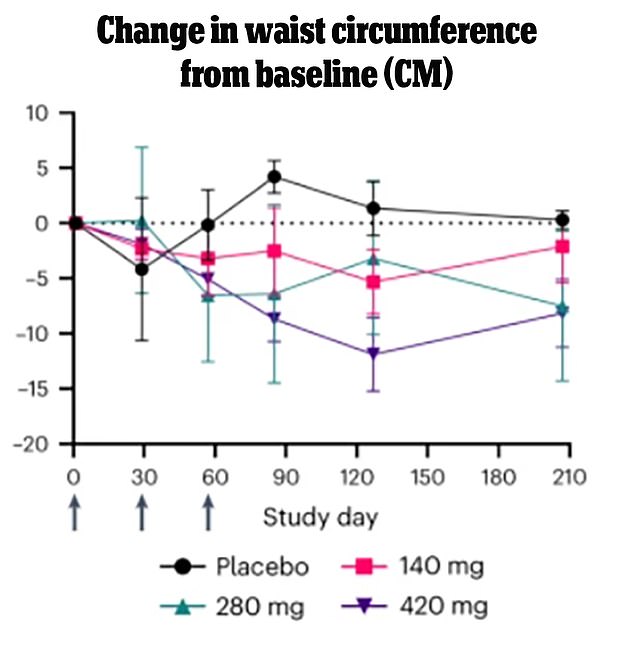
People who received the highest dose of the monthly injection saw a steady decrease in waist circumference. The variability in centimeters lost reflects the large variability in such a small sample size of patients. They also saw continued decline after the shots stopped
Their weight then started to increase slowly, but their body weight was still 11 percent lower five months after the last dose.
Narimon Honarpour, senior vice president of global development at Amgen, said: “That’s a really remarkable and distinctive feature of this molecule.”
If subsequent phases of the study strengthen early results, Amgen’s drug will inject a dose of competition into the growing anti-obesity drug market currently dominated by Novo Nordisk’s blockbusters.
Wegovy, and to a lesser extent its sister drug Ozempic approved for the treatment of obesity-related type 2 diabetes, is extremely difficult for patients to obtain due to excessive demand that exceeds anemic supply.
Insurance companies are also loathe to cover Wegovy because of its exorbitant sticker price – as much as $16,000 per year.
That doesn’t stop people from taking the photos.
In a study conducted in the fall of 2023 by the online physician community platform Sermo, which surveyed 346 healthcare professionals, 89 percent reported seeing a recent increase in patients seeking weight loss medications, while 92 percent of primary care physicians said they had actively prescribed such medications.
