Scientists identify a NEW key cause of female infertility
- Experts claim to have discovered why some women struggle to conceive
- Researchers think that a genetic change causes the function of egg cells to change
- READ MORE: EXCLUSIVE Question about 'unvaxxed' sperm spikes
Scientists have discovered a new fundamental cause of female infertility.
Changes in a specific gene called Eif4enif1 have been identified as the cause of a problem with the ovaries that makes fertilization almost impossible.
Researchers from Tsinghua University in China have discovered that the telltale genetic change causes a problem with the eggs, preventing the ovaries from releasing eggs regularly.
Without the release of eggs – the ovulation process – fertilization cannot occur.
Specifically, the harmful DNA pattern affects the functioning of the mitochondria in the egg cells – the 'powerhouse' that converts fuel into energy.
The fertility rate among American women has been slowly declining for years. Infertility affects approximately 48 million couples worldwide, and approximately one in five women in the US is infertile
Infertility affects approximately 48 million couples worldwide, and approximately one in five women in the US is infertile.
In about one to two percent of cases, the problem is caused by the ovaries' failure to release an egg.
Speaking about the findings, Professor Kehkooi Kee from Tsinghua University in China, who helped lead the study, said there is a link between Eif4enif1 and mitochondria had not been previously identified.
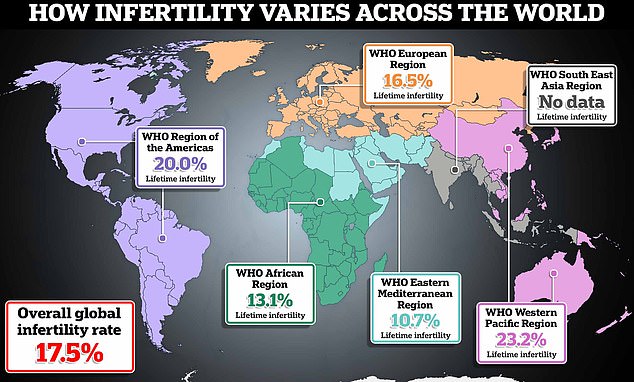
The WHO estimates that one in six adults worldwide will experience infertility during their lifetime. There are regional differences. The Eastern Mediterranean recorded the lowest infertility rate at just 10.7 percent, followed by Africa at 13.1 percent and then Europe at 16.5 percent. The Western Pacific recorded the highest rate of 23.2 percent, followed by the Americas at 20 percent. No figure was available for the Southeast Asia region due to a lack of quality studies in that area
Medically, problems with egg production and release are known as both primary ovarian insufficiency and premature ovarian insufficiency.
The term premature refers to the fact that the problem occurs before menopause, when ovulation naturally stops.
In 2019, Chinese researchers came across a family with premature ovarian insufficiency, all of whom had changes in the Eif4enif1 gene.
The researchers reproduced this genetic change in mice to see how it affects human fertility.
They grew the mice and compared their fertility with mice whose DNA had not been modified.
They found a characteristic pattern of changes in egg mitochondria.
The average number of total follicles – the small sacs on the surface of the ovaries that contain developing eggs – was reduced by about 40 percent in older and genetically modified mice.
The average number of baby mice per litter also fell by a third.
And when grown in a dish, about half of the fertilized eggs did not survive beyond the early stages of development.
When the researchers examined eggs from the less fertile mice under a microscope, they found that the mitochondria were not evenly distributed throughout the egg, as they should be, but were in clusters.
It appeared that the misbehaving mitochondria contributed to the mice's infertility.
The researchers think that restoring proper mitochondrial behavior could improve fertility.
Their next steps will be to see if mitochondrial defects are also seen in the eggs of human patients.
The findings also suggest there is potential for gene editing drugs that could tackle the problem and provide an effective treatment.
The research was published in the journal Development.
