Scientists grow the world’s first artificial human TESTICLES in a laboratory, marking a potential breakthrough
Scientists have announced that they have grown artificial testicles in a dish, a development they say could help treat infertility in men.
These lab-grown testicles are not yet fully functioning, sperm-producing organs, but they do share many of the same structures and genetic characteristics as natural ones.
This will allow scientists to investigate and possibly treat fertility problems in men by artificially producing sperm.
Additionally, the scientist who led the work told DailyMail.com that she believes these artificial testicles could be used to study the effects of various toxins on testicular function.
Research has increasingly suggested that environmental pollutants in everything from food to children’s toys affect male fertility, and many believe the rise of these chemicals is partly fueling America’s fertility problem.
This testicular organoid was incubated in a laboratory dish for 14 days. The tubular structures are a crucial part of the testicular anatomy, indicating that this organoid functions as expected
And later, the technique used to grow these artificial testicles could help survivors of childhood cancer have children of their own if chemotherapy makes them infertile.
“We plan to create human testicular organoids and investigate whether sperm can be generated there,” said lead researcher Nitzan Gonen, a researcher at Bar Ilan University in Israel.
Artificial sperm cells would presumably have faithful copies of a man’s DNA, because they would be grown from his own stem cells.
Fertility problems affect 10 to 15 percent of men, and male infertility plays at least a partial role in half of all cases of couples failing to conceive.
But it is difficult for scientists to study the reasons behind male infertility because there is currently no laboratory model to study the testicles.
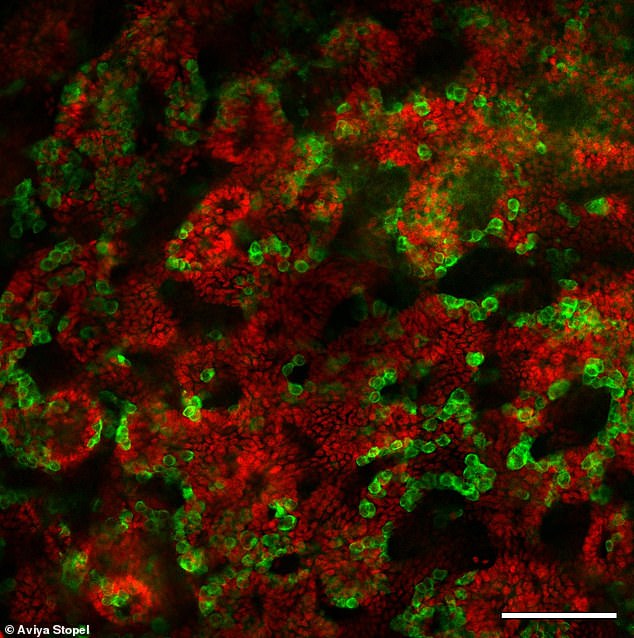
These testicular organoids were grown from mouse pup cells and incubated in a dish for 21 days. Sertoli cells (red) are responsible for the formation of the tubules in the testicle. Germ cells (green) will produce the sperm cells.
That’s where these testicular organoids come in, miniature 3D models of testicles that can be used to study the real thing.
In the past, scientists had to rely on data collected from the testicles of deceased people, which do not provide the same insights as these organoids, which are not alive but continuously growing as if they were still alive.
The artificial testicles in this study started out as stem cell samples taken from testicles of newborn or embryonic mice.
Stem cells have the potential to develop into any of the body’s many cell types, including brain, muscle, skin and testes.
To grow the testicles, Gonen’s team placed these stem cell samples in laboratory dishes and allowed them to develop into the different types of cells that make up mouse testicles.
There they grew for several weeks, and scientists periodically examined them to see if they resembled naturally grown mouse testicles.
The result is not exactly testicles, but rather structures called “testicular organoids,” meaning small versions of the organs that contain the same types of cells as the original and behave in the same way.
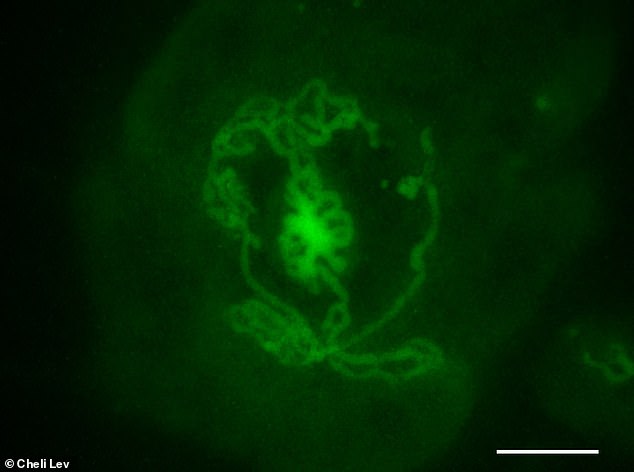
This testicular organoid is made from mouse embryos and incubated in a dish for 14 days. The tubular structures are the same. Highlighted in green are Sertoli cells, the cells responsible for forming the tubules in the testicle and which indeed create the tubules in the shell.
Organoids look a lot like organs in the embryonic stage of development, Gonen said.
After eight or more weeks, the testicular organoids showed signs of entering meiosis, the stage in the testicles’ life cycle where they produce sperm cells.
Testicles are made up of a number of different cell types that perform multiple functions, including producing sperm and releasing hormones.
Crucially, they found that testicular organoids could be grown from newborn or embryonic mice, but not from adults.
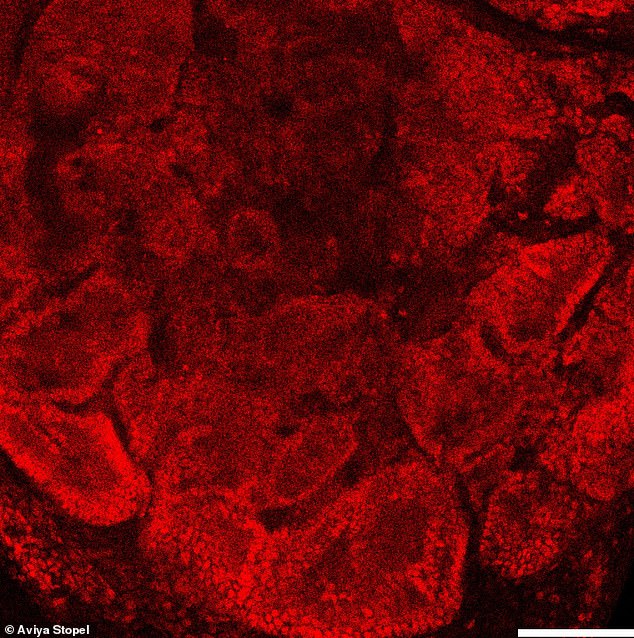
These 21 day old cells are located in testicular organoids. They are called Sertoli cells and they grow the tubule cells needed for the testicles to function
Examining the organoids of the testis, Gonen and her team found that they contained some of the essential structures for normal testicular function: tubules, germ cells and Sertoli cells.
The tubules transport sperm, the sex cells produce the sperm cells, and the Sertoli cells help form the tubules.
These were all present in the testicular organoids.
The study appeared in the International Journal of Biological Sciences.
“We still can’t say for sure whether our organoids enable full sperm production in the dish, but we saw signs that the germ cells in the organoids we generated are entering meiosis,” Gonen said. Meiosis is the process by which sperm halves its DNA in preparation for fertilization of an egg.
‘We are now working to better understand whether we have been successful in producing sperm.’
The team is now investigating whether these organoids can actually produce sperm cells – and whether they can produce sex hormones such as testosterone.
They also plan to see if they can grow similar artificial testicles from biopsies of pre-pubescent boys about to undergo chemotherapy.
Gonen said she and her team also want to grow “completely artificial testis” from stem cells, not just testicle cells.
