Scientists develop mini brains made from aborted fetuses that could revolutionize neuroscience
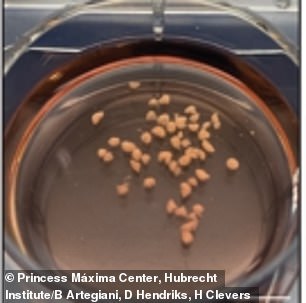
Instead of using individual cells, scientists used small pieces of fetal brain tissue to grow mini brains, which were about the size of a grain of rice
Scientists have developed the world's first mini-brain, a breakthrough they hope could revolutionize neuroscience.
The 3D organs – which are about the size of a grain of rice – were grown in a laboratory using human fetal brain tissue from healthy abortion material.
Scientists at the Princess Máxima Center for Pediatric Oncology in the Netherlands tried to grow brains in early to middle development and therefore used the brain tissue of an aborted fetus that was in the gestational period of weeks 12 to 15.
The team was surprised to discover that fetal brain tissue was essential for the growth of a mini brain.
Until now, when growing other mini-organs, scientists broke down original tissues into individual cells by using embryonic or pluripotent stem cells to grow and replicate specific parts of the brain.
This discovery led scientists to consider using the mini-organ to model brain cancer and focus specifically on how it develops in children, in the hope that it will lead to a cure.
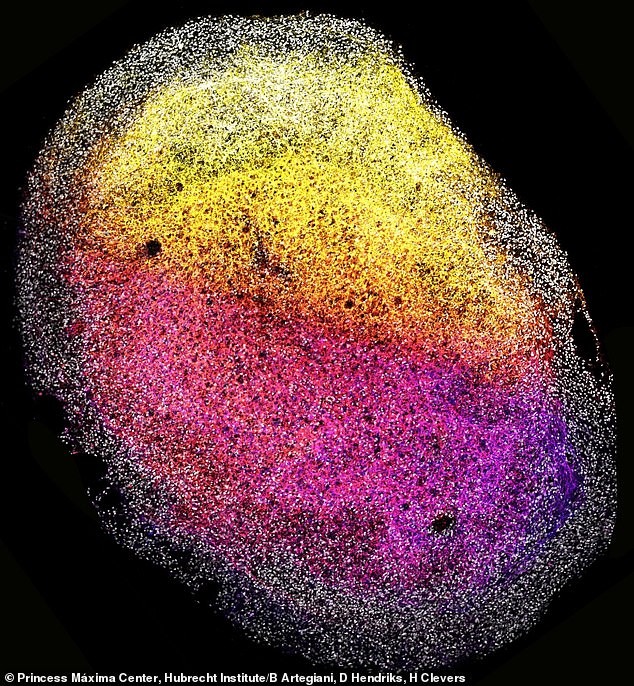

The mini-brain (shown close-up) is about the size of a grain of rice and can be multiplied for future research efforts
“These new organoids derived from fetal tissue can provide new insights into what shapes different brain regions and what creates cellular diversity,” said Dr. Delilah Hendriks, group leader at the Princess Máxima Center for Pediatric Oncology, postdoctoral researcher at the Hubrecht Institute and Oncode Investigator, who co-authored the study.
Anonymous donors agreed that scientists could use the tissue for research purposes only.
The researchers wanted to know if they could create a long-term expanding organ with the same cellular complexity as the human brain.
Using the fetal brain tissue, the team cut the tissue into small pieces and organized them in a dish before placing the cultures on an orbital shaker.
In the first four to eight days, researchers noticed that multiple 3D structures formed and continued to grow, while still resembling brain tissue in appearance.
The team found that by splitting the organoid in the fetus' brain, it would reform to twice its original size within 20 to 30 days of culture.
This entire process took eight months, with the mini-brain eventually growing to 1,500 cubic centimeters. At that point, growth slowed but could be maintained.
The team then focused on its primary goal: fighting brain cancer, using some of the newly formed organoids to develop mini-tumors.
The team used CRISPR-Cas9, a gene editing technique, to introduce a small number of cells into the organoids to introduce a cancer gene called TP53 to model brain cancer.
Crispr-Cas9 is a tool for making precise edits to DNA discovered in bacteria.
It took three months for the gene to fully take over healthy cells, replicating the same features of typical cancer cells and demonstrating the potential for cancer drug research.
Their findings, published in the peer-reviewed journal Cellrevealed that it could be possible to use the organoids to search for a cure for cancer and 'could revolutionize brain research.
“Brain organoids from fetal tissue are an invaluable new tool to study human brain development,” said co-author Dr. Benedetta Artegiani.
'Our new tissue-derived brain model allows us to gain a better understanding of how the developing brain regulates cell identity.
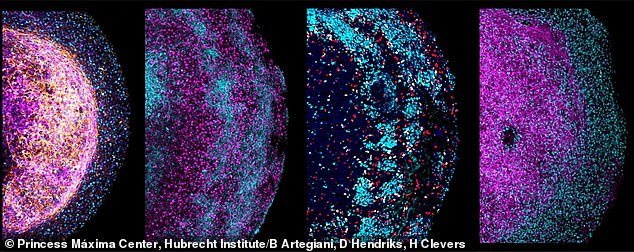

Four zoomed-in images of parts of different human fetal brain organoids. Several neural markers are colored, depicting their cellular heterogeneity and architecture
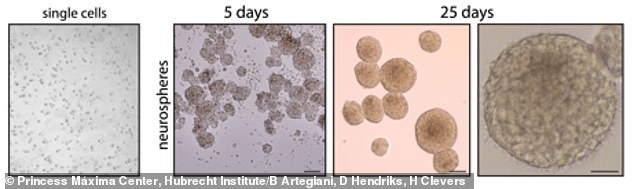

The team shared images showing how the tissues grew over the course of 25 days
'It could also help understand how errors in that process can lead to neurodevelopmental diseases such as microcephaly, as well as other diseases that can arise from derailed development, including brain cancer in children.'
Nearly 16,000 children between birth and 19 years old are diagnosed with cancer each year in the U.S., 4,000 of whom are diagnosed with brain and spinal cord tumors.
The brain and spinal cord tumors are responsible for about one in four cases of cancer in children, and about one in four children diagnosed with brain cancer do not survive, according to the American Cancer Society.
The Princess Máxima Center therefore says it is driven to find a solution and believes that successfully developing the mini brain will bring scientists one step closer to a cure.
'We work together every day with passion and without boundaries to improve the survival rate and quality of life of children with cancer. Now and in the longer term, Princess Máxima Center says: 'Because children still have their whole lives ahead of them.'
