Patients’ fears as FDA delays decision to approve Eli Lilly’s ‘life-changing’ Alzheimer’s drug – over concerns about deadly side effects and how drugs were tested
An unexpected move by the FDA to delay approval of a potentially life-changing Alzheimer’s drug has left doctors and patients in the lurch who were eagerly awaiting its availability.
Eli Lilly, the maker of the drug known as donanemab, said its approval would be delayed until after March as the FDA plans to convene a last-minute meeting of outside experts to review its safety and efficacy. judge.
The drug was expected to be approved by the end of the first quarter of the year after clinical trials showed it could slightly slow the rate of cognitive decline associated with the disease.
But the FDA wants an independent opinion on the drug’s main side effect: It can cause fatal swelling of the brain.
Donanemab was shown to slow cognitive decline by 60 percent in patients with early stages of the disease
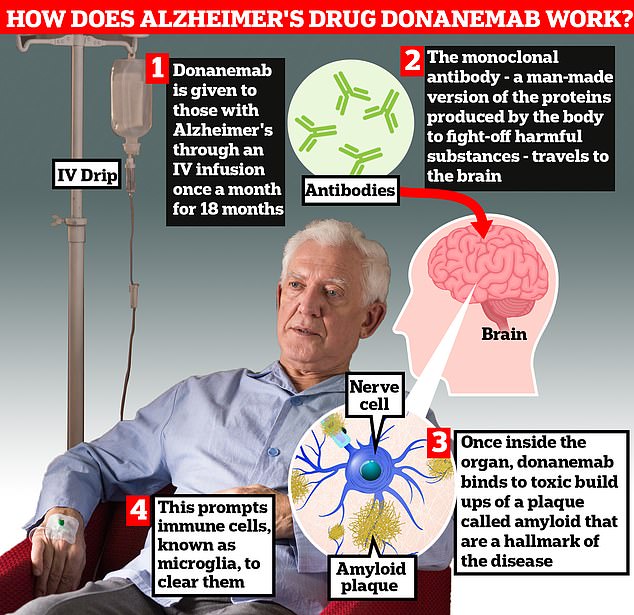
Donanemab is given to Alzheimer’s patients once a month through an IV infusion. The monoclonal antibody – a man-made version of proteins produced by the body to fight harmful substances – travels to the brain. Once inside the organ, donanemab binds to the toxic build-ups of amyloid plaque – a hallmark sign of the memory-robbing disease. This causes immune cells known as microglia to clear them away
The FDA wants outside experts to examine the company’s unique trial design, which allowed patients to stop taking the drug once plaques believed to cause Alzheimer’s disease were cleared from the brain.
The move is a setback for Alzheimer’s patients and their advocates, who are urging the FDA to take swift action and do everything it can to approve the drug.
Professor Robert Howard, an expert in geriatric mental health at University College London, said: ‘This is very bad news for Lilly.
“The longer (competing) lecanemab is licensed, but donanemab is not, the more the available (but limited) U.S. capacity to treat patients with amyloid antibodies (18 months) will be blocked by the first drug to hit the market.”
Donanemab belongs to a growing class of drugs aimed at reducing clumps of a sticky protein called beta-amyloid, which is thought to be the main cause of this specific type of dementia.
In the company’s clinical trial, the monthly injection, 60 percent of patients showed a slowing of cognitive decline after one year of use based on criteria from one type of symptom rating scale, and by 46 percent according to another scale.
Among patients with early Alzheimer’s disease whose brain scans showed low or moderate levels of a protein called tau, the drug was found to slow clinical decline by 35 percent.
Levels of tau are another indicator of how far the disease has progressed. There is debate in the field of dementia research as to whether amyloid buildup is the cause of the disease or whether it is tau.
This protein undergoes abnormal changes in the brain and becomes entangled with other protein strands. The build-up of this tangle prevents brain cells from communicating effectively with each other.
The FDA’s decision was a blow to advocates like the Alzheimer’s Association.
A spokesperson for the advocacy group said: ‘While the decision at this stage of the review process is a surprise, safety and the correct administration of treatments is paramount.
“On behalf of all who could benefit from this treatment, we strongly urge the FDA to take swift action in this next phase of its review.”
The drug, as well as a similar treatment Leqembi from Biogen, is intended for people with mild cognitive impairment in the early stages of Alzheimer’s disease. About six million Americans have the disease, and about 300,000 are in the early stages and are likely candidates for the drug.
The field of Alzheimer’s treatment development has been plagued by safety failures, chief among them brain bleeding and swelling, and by doubt that they would actually work.
Previous studies have shown only a mild decrease in the rate at which the disease causes the mind to waste away, which has sown doubt in the minds of reviewers charged with approving or rejecting the drug companies’ applications.
Still, ample evidence shows that some of the nearly 1,800 people who participated in Lilly’s trial benefited from the drug, including Connecticut native Jim Sirois, who is now 68.
In 2020, he was diagnosed with early-onset Alzheimer’s disease after struggling to speak and becoming so forgetful that he couldn’t remember where he had been the day before. But his wife said the drug effectively stopped the condition over the course of 2023.

Jim Sirois and his wife Sue. Jim was diagnosed with early-onset Alzheimer’s disease more than three years ago and since starting donanemab, his disease has ‘really not progressed’

Myra Garcia of Southern California, pictured above with her husband Richard, also received the drug after being diagnosed with early-onset Alzheimer’s. She told DailyMail.com that everyone who gets the chance should take the drug
Sue Sirois, 64, said: ‘I liken Jim’s decline to a friend I know who was diagnosed with Alzheimer’s at the age of 56. She died recently and only lasted six years, while Jim is now over three years later and still looking beautiful today. pretty much the same as what he was.”
Other patients in the trials of the drug were happy that it helped them continue to do “normal things” like going to class and doing laundry.
Myra Garcia, 64, of Southern California, is also receiving donanemab after being enrolled in a separate study. She told DailyMail.com that she would get the drug approved tomorrow if she could, adding that it has “worked beautifully.”
She added, “I’m in three choirs: my church choir, the Hillcrest choir and a Bach choir.
‘I also exercise with my husband, we do something together every morning, but then I cook and wash and the things everyone does.’
