Nurse’s death is first in the UK to be linked to NHS-approved weight loss jab Mounjaro after 58-year-old fell ill taking two low-dose injections
The death of a nurse has been linked to the use of a weight loss drug approved for use by the NHS.
Susan McGowan, 58, from Lanarkshire, died of multiple organ failure, septic shock and pancreatitis after taking two low dose injections of tirzepatide, known by the brand name Mounjaro.
She took the jabs over a two-week period before her death on September 4, believed to be the first death officially linked to drugs in Britain.
Ms McGowan had been a nurse at Monklands University Hospital in Airdrie, Scotland, for more than 30 years and had often talked to her friends about her struggle to lose weight.
Just days after taking the drug, which costs between £150 and £200 for a four-week supply, Ms McGowan developed severe stomach pains and went to the emergency room at the hospital where she worked.
Although her colleagues fought to save her, she tragically died with her niece Jade Campbell by her side.
Earlier this year it was revealed that the medicines watchdog received reports of ten deaths linked to the use of slimming shots and 7,228 reports of nausea, vomiting and diarrhea associated with products such as Wegovy and Ozempic.
Susan McGowan, 58, (left) died with her niece Jade Campbell (right) by her side two weeks after taking a weight-loss drug

The thirty-year-old nurse went to the emergency room of the hospital where she worked with severe abdominal pain, but unfortunately her colleagues could not save her

Weight loss jab Mounjaro was approved for use by NHS Scotland by the Scottish Medicines Consortium in June this year
Of these, 68 patients were admitted to hospital, the Medicines and Healthcare products Regulatory Agency (MHRA) said.
The figures are based on users or health professionals telling regulators about side effects of the drugs, known as glucagon-like peptide-1 receptor agonists (GLP-1RAs).
A reported death or adverse event does not necessarily mean it was caused by the drug, just that someone suspected it might have been the case.
Underlying or concurrent diseases and other medications that the patients may have been taking at the time of their death may be responsible and such events may also be coincidental, the journal Chemist and Druggist said.
The MHRA has urged healthcare professionals to ‘report cases of misuse’ and ‘inform patients of the common and serious side effects associated with GLP-1RAs’.
At the time, the company said it was aware of 46 hospital admissions on August 16 – suggesting there had been a further 22 reports in two months, representing a 48 per cent increase.
Between January and May this year, there were 208 reports of tirzepatide under the Yellow Card, which records the adverse effects of medicines in Britain.
Such side effects included 31 serious reactions and the suspected death of a man in his 60s.
Ms McGowan purchased a prescription through a registered online pharmacy after researching Mounjaro and seeking medical advice.
Her niece Jade told her BBC: ‘Susan had always been a little bit extra heavy, but there were never any health problems. She was taking no other medications. She was healthy.’
‘Susan was such a vibrant person. She was very generous, she was very kind and she was the life of the party – a huge personality. They said she had the biggest laugh in the hospital.”
In June, Scotland became the first country in Britain to approve Mounjaro, which has been dubbed the ‘King Kong’ of obesity jabs.
The Scottish Medicines Consortium ruled this year that Mounjaro could be made available to people through the NHS to help them lose weight.

Mrs McGowan was described by her niece Jade as a ‘bubbly’ person and the ‘life of the party’
Mounjaro is the latest in a line of slimming injections that have helped users lose massive amounts of weight, and is considered one of the most effective.
It costs the Scottish NHS £33.6 million a year. The cost of obesity to the NHS is estimated at £600 million per year.
Mounjaro is the brand name of a drug called tirzepatide and is given as a weekly injection in different strengths.
It is the latest in a new generation of jabs that help people lose weight, similar to Ozempic and Wegovy which have also recently been approved by the SMC.
The drugs, known as glucagon-like peptide 1 receptor agonists (GLP-1 RAs), suppress hunger by mimicking hormones that signal the body is full.
Mounjaro also slows the passage of food through the stomach.
Research has shown that people can lose up to 20 percent of their body weight in 36 weeks by using Mounjaro.
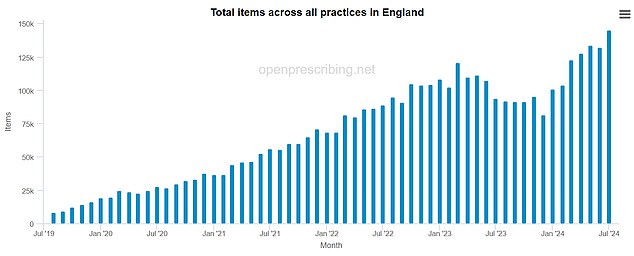
NHS-backed data source OpenPrescribe shows rising prescriptions for semaglutide, the drug in Ozempic and Wegovy
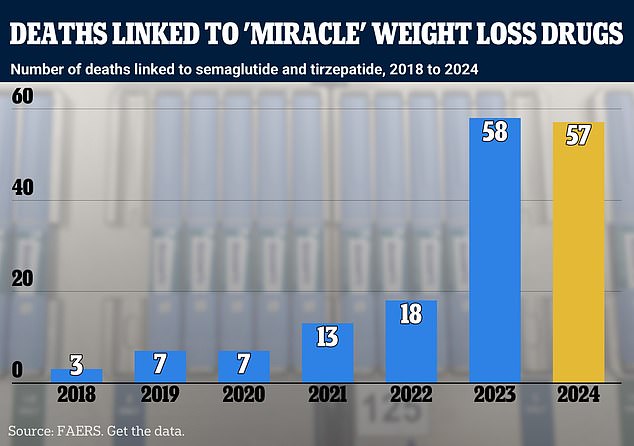
Deaths in America associated with semaglutide and tirzepatide per year. Semaglutide is the active ingredient in Ozempic and Wegovy, while tirzepatide is used in drugs including Zepbound. For 2024, yellow is used to indicate that the data is incomplete
The difference in results prompted American diabetes expert Dr Julio Rosenstock to declare Mounjaro ‘King Kong’ compared to ‘the gorilla’ of his rival Wegovy.
However, in general, people should continue taking the medicine to maintain their weight loss.
Mounjaro was recently approved by the SMC as a treatment for type 2 diabetes as it also helps stimulate the production of insulin, the hormone that regulates blood sugar levels.
The June ruling cleared the way for thousands of Scots without diabetes to receive injections to help them lose weight.
Earlier this week, the Danish manufacturer of the famous weight-loss shot Ozempic revealed that ten Americans died and 100 were hospitalized after taking pharmacy-made counterfeits of their drug.
Meanwhile, wEight loss shots like Ozempic have been linked to 162 deaths in the US, DailyMail.com revealed.
One of the victims was a 45-year-old woman who choked on her own vomit while taking Mounjaro, a rival drug that works in the same way.
Another involved a 23-year-old man who died of vomiting, nausea and a rapid heartbeat after taking Wegovy.
