New pill-sized device that monitors breathing from INSIDE the body to detect opioid overdoses successfully clears human trials
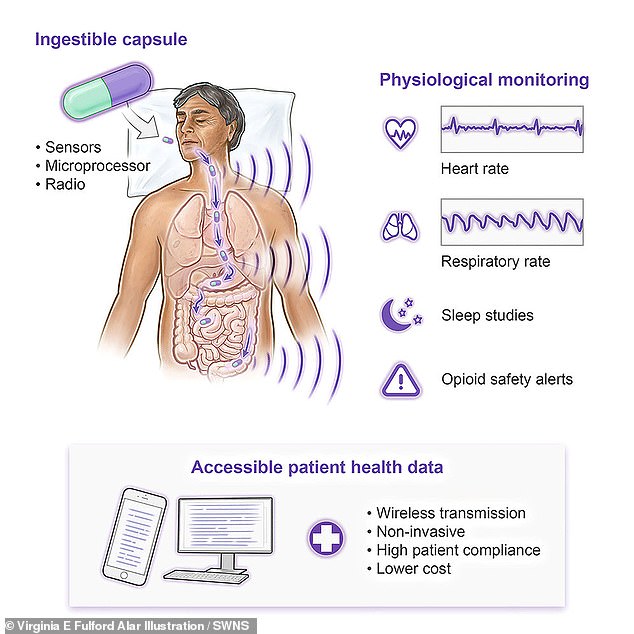
A new pill-sized device that can safely monitor life-saving vital signs from the stomach has successfully passed its first human tests.
The swallowable device is designed to monitor vital functions such as breathing and heart rate from within the body.
Scientists say the device, described in the journal Device, has the potential to provide life-saving care to people at risk of opioid overdose.
The team hopes to expand its use to monitor other health problems, such as sleep disorders.
Lead author Professor Giovanni Traverso, a gastroenterologist at Brigham and Women’s Hospital in Massachusetts, said: ‘The stomach generally gives some of the best signals, especially because it is close to the heart and lungs, but we know we can’t get them. can also observe elsewhere. .
‘The ability to facilitate diagnosis and monitor many conditions without having to go to a hospital can provide patients with easier access to healthcare and support treatment.’
Unlike implantable devices such as pacemakers, ingestible devices are easy to use and do not require surgical intervention, and doctors have been using pill-sized ingestible cameras for years to perform procedures such as colonoscopies.
The team tested the device in humans by giving it to people being assessed for sleep apnea – a condition in which breathing repeatedly stops and starts during sleep.
Professor Traverso says the current version of the VM pill passes through the body through the stool in about a day, but there are changes the researchers can make to the device in the future that could allow it to remain in the body for longer. term monitoring.
The device has the potential to help tens of thousands of people who overdose on opioids every year. The number of overdose deaths involving opioids rose to 80,411 in 2021, the highest ever since 1999.
Deaths involving prescription opioids reached 16,706 in 2021, down about 320 from a high of 17,029 in 2017.
Unlike implantable devices such as pacemakers, ingestible devices are easy to use and do not require surgery, and doctors have been using pill-sized recordable cameras for years to perform procedures such as colonoscopies.
Co-author Benjamin Pless, founder of medical device developer Celero Systems, said: ‘The idea of using a swallowable device is that a doctor can prescribe these capsules, and all the patient has to do is swallow them. to swallow.
“People are used to swallowing pills, and the cost of using swallowable devices is much cheaper than performing traditional medical procedures.”
He explained that the vital signs monitoring pill, or VM Pill, works by monitoring the body’s small vibrations related to breathing and the beating heart, and that the pill can detect if a person stops breathing from the digestive tract.

Lead author Professor Giovanni Traverso said: ‘The stomach generally gives some of the best signals, especially as it is close to the heart and lungs, but we know we can sense them elsewhere too’
To test the VM Pill, researchers placed the device in the stomachs of pigs while the animals were under anesthesia.
The team then administered a dose of fentanyl to the pigs, which caused the animals to stop breathing, similar to what happens in humans when someone overdoses on the drug.
The device measured the pigs’ breathing in real time and alerted the researchers when the animals stopped breathing. The team was then able to reverse the overdose.
The team then tested the device in humans for the first time by giving the VM pill to ten people being evaluated at West Virginia University for sleep apnea – a condition in which breathing repeatedly stops and starts during sleep.
The patients showed no adverse effects from swallowing the capsule, which passed unnoticed through their digestive tract.
Mr Pless said: ‘Given our interest in the safety of opioids, we have noticed that sleep apnea shares many of the same symptoms as opioid-induced respiratory depression.’
The device was able to detect when the participants’ breathing stopped and could monitor breathing rate with 92.7 percent accuracy.
Compared to external vital monitoring machines, the pill can monitor heart rate with at least 96 percent accuracy.
The trial also showed that the device is safe, and all participants excreted the device in their stool a few days after the experiment.
Co-author Dr Ali Rezai, a neuroscientist at West Virginia University, said: ‘The accuracy and correlation of these recordings were excellent compared to the clinical gold standard studies we conducted in our sleep laboratories.’
He added, “The ability to remotely monitor critical vital signs of patients without wires, cords or the need for medical technicians opens the door to monitoring patients in their natural environment versus the clinic or hospital environment.”
The team hopes to upgrade the device so that it can automatically deliver medications to reverse conditions such as opioid overdose as soon as the device detects symptoms.
Professor Traverso added: ‘Going forward, there are many situations, including opioid overdose and other respiratory and cardiac conditions, that could certainly benefit from this ingestible device.’
