New malaria vaccine is only the 2nd to win an endorsement from the World Health Organization – and this shot is more effective and less expensive
The World Health Organization has given the green light to a new, more effective vaccine against malaria, the first vaccine to date to meet the organization’s efficacy standards.
Malaria, a mosquito-borne disease, killed about 619,000 people worldwide in 2021 and the number of global cases exceeded 247 million, up from 245 million the year before. 96 percent of those cases were recorded in the WHO’s African region, where the disease is endemic. then 1 billion people will live there.
The global health agency is now recommending the low-cost, University of Oxford-designed R21/Matrix-M vaccine, only the second malaria vaccine approved by the WHO and the first to reach the agency’s 75 percent efficacy standard.
The first vaccine to win the WHO’s recommendation was the RTS vaccine in 2021 and despite what the WHO calls “unprecedented” demand for shots, supplies are extremely limited, with only around 18 million doses allocated for the next two years .
Although this vaccine received the support of the WHO, it did not meet the organization’s efficacy standards. However, it is still used to vaccinate people against malaria.
The approval this week marks a major step forward in the WHO’s mission to eradicate malaria from at least 30 countries by 2030, but with the first wave of vaccinations not available until mid-2024, the disease will have plenty of time to kill lives. of endangering people. millions.
The graph shows the incidence of malaria cases, or cases per 1,000 people at risk, over time. The incidence of malaria cases fell from 82.3 per 1,000 in 2000 to 57.2 in 2019, before rising by around four percent in 2020
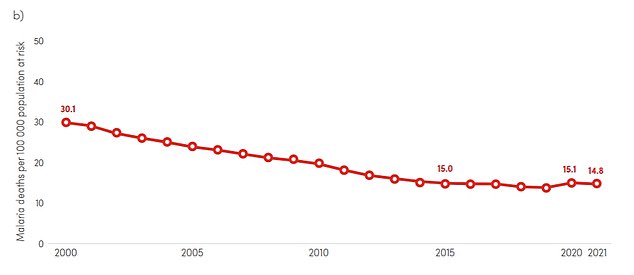
The graph above shows the number of deaths per 100,000 people at risk of malaria. The death rate halved between 2000 and 2015 and continues to decline
About 40 million children live in countries affected by malaria and in 2021, 80 percent of malaria cases detected in the African region were among children under the age of five, up from 72 percent in 2015.
Children, as well as pregnant women and travelers who do not have basic immunity against the malaria-causing parasite, are at greatest risk of serious infections and death.
The new vaccine was tested in five locations in Burkina Faso, Mali, Kenya and Tanzania, where malaria outbreaks occur seasonally, with most cases occurring between September and May.
The newer R21 vaccine was found to reduce symptomatic cases of malaria by 75 percent over the 12 months following a three-dose series of injections.
Like the 2021 RTS vaccine, the R21 vaccine requires a booster after twelve months to maintain protection.
The R21 shots also show an improved ability to protect people from the disease for a longer period of time than the older vaccine.
With a longer period of protection, young children are much less likely to get sick and even less likely to experience symptoms such as fever, chills, headache, nausea, joint pain and rapid heartbeat.
The older RTS injection’s effectiveness in preventing disease in children aged five to 17 started at 74 percent, but a year later the protective effect had dropped to 28 percent. In infants aged six weeks to three months, this fell to 11 percent a year later.
With the new vaccine, there was still a 66 percent efficacy in preventing malaria after a year, and giving a fourth dose of the R21 shot a year after the third prevented waning immunity.
Clinical trials have also shown the new vaccine to be safe.
Dr. Tedros Adhanom Ghebreyesus, Director-General of WHO, said: “As a malaria researcher, I dreamed of the day when we would have a safe and effective vaccine against malaria. Now we have two.
‘Demand for the RTS vaccine (approved in 2021) far exceeds supply, so this second vaccine is an essential additional tool to protect more children faster and move us closer to our vision of a malaria-free future.’
Malaria infection occurs when a female Anopheles mosquito carrying the Plasmodium parasite bites an unsuspecting victim, transmits the parasite to them and then travels to the liver, where it can remain inactive for up to a year.
When the parasite matures, it leaves the liver and poisons the bloodstream. Taking medications to kill the parasite early in the infection resolves the problem in about 93 percent of cases. But if left untreated, the condition is almost always fatal.

The female Anopheles mosquito becomes infected with the malaria-causing parasite. When it bites a human, the parasite matures in the body until it exhibits symptoms such as fever, joint pain and, in most cases where left untreated, death.
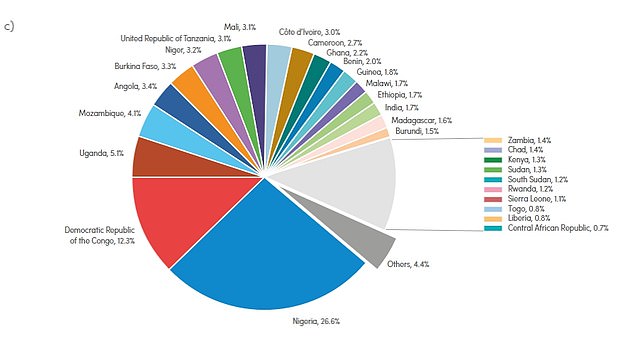
The WHO’s African region was responsible for about 95 percent of malaria cases and 96 percent of deaths worldwide between 2019 and 2020
Cases in the U.S. occur in sporadic clusters, with about 2,000 reported in the country each year, largely associated with international travel to destinations where malaria is common.
This year there have been seven cases of malaria in Florida and one each in Texas and Maryland.
The burden is heavier in Africa and tropical regions of Asia, where climate is fueling a booming population of Anopheles mosquitoes and demand for effective and cheap vaccinations has long lagged.
The Serum Institute of India (SII), one of the world’s largest vaccine makers, has ensured it can produce 100 million doses per year. That percentage will double in the next two years.
And at a price of $2 to $4 per injection, “the cost-effectiveness of the R21 vaccine would be comparable to other recommended malaria interventions and other childhood vaccines,” the WHO said.
The costs of the RTS recording are average about $5 per unit.
Dr. Matshidiso Moeti, WHO Regional Director for Africa, said: “This second vaccine has real potential to close the huge gap between supply and demand. If widely supplied and rolled out, the two vaccines could contribute to the prevention and control of malaria and save hundreds of thousands of young lives in Africa from this deadly disease.”
