Multiple sclerosis medication could potentially treat Alzheimer’s disease, study suggests, and the approved drug could be available to patients immediately
Ponesimod, sold under the brand name Ponvory, is currently approved for the treatment of relapsing forms of MS
A drug for multiple sclerosis (MS) could hold the key to treating Alzheimer’s disease, a first study of its kind suggests.
Researchers recently found that ponesimod, a drug already approved by the Food and Drug Administration (FDA) to treat relapsing forms of MS, reduces inflammation in the brain — a key component of Alzheimer’s progression.
Neuroscientist Erhard Bieberich, principal investigator of the study at the University of Kentucky, said: “We are the first to show that ponesimod is effective in a mouse model of Alzheimer’s disease.”
Mr Bieberich added that because ponesimod, which is sold under the brand name Ponvory, is already approved for MS, the process of getting the drug into Alzheimer’s patients is both cheaper and easier.
This is because the drug has already been tested for safety in humans, meaning it could get it to market faster compared to a new drug that would have to go through the traditional FDA review process, which can take years.
It is estimated that 6.2 million Americans age 65 and older are living with the disease, a progressive and irreversible neurological disorder that affects cognitive function, memory and behavior.

Zhihui Zhu (left) and Erhard Bieberich (right) teamed up with fellow researchers to study the FDA-approved drug ponesimod as a potential therapy for Alzheimer’s disease
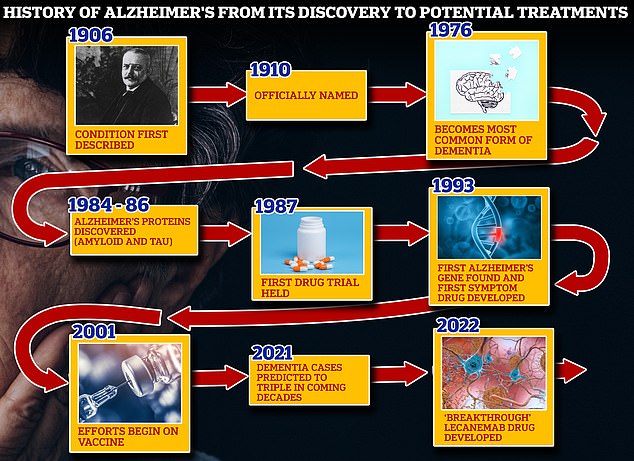
From 1906, when clinical psychiatrist Alois Alzheimer first reported a “serious disease of the cerebral cortex,” to the discovery of the disease’s mechanisms in the 1980s and 1990s, scientists have spent more than a century trying to tackle the brutal disease. suits that rob people of their cognition. and independence
MS is a potentially disabling disease of the brain and spinal cord in which the immune system attacks the protective covering of nerve fibers, causing communication problems between the brain and the body.
Malfunctioning of the immune system causes inflammation, a major cause of many MS symptoms and Alzheimer’s disease.
In MS patients, Ponesimod, a pill taken once a day, works by calming inflammation in the brain.
In Alzheimer’s patients, a protein called beta-amyloid peptide accumulates in the brain and disrupts brain cell function. Microglia, a type of immune cell, are needed to remove the clumps of beta-amyloid.
The researchers focused on microglia, which regulate inflammatory responses in the brain and spine, because dysfunctional microglia are linked to neurodegenerative diseases, such as Alzheimer’s disease.
While microglia are supposed to help clear the buildup of the beta-amyloid peptides, the buildup disrupts communication between the brain’s nerve cells when they malfunction, eventually causing them to die.
Because of the similarities between Alzheimer’s disease and MS, the researchers wanted to see if ponesimod could stop the inflammation that causes Alzheimer’s disease.
They used mice genetically engineered to display human-like features of Alzheimer’s disease, as well as post-mortem samples of brain tissue donated by Alzheimer’s patients.
“The clearance of those proteins is an important target for treating Alzheimer’s disease,” said Zhihui Zhu, Ph.D., first author of the study and one of the scientists in Bieberich’s lab.
In the study, the researchers ‘reprogrammed’ microglia into cells that helped clear the toxic proteins in the brain.
The results were “promising,” with signs that the MS drug reduced inflammatory responses that could be responsible for Alzheimer’s disease, and encouraged microglia to eliminate protein clumps and tangles in the brain.
While the results are encouraging, the drug’s effectiveness in humans has yet to be proven.
The findings were published in the journal eBioMedicine last month.
