A new vaccine that can completely block the effects of fentanyl will soon enter human clinical trials.
The injection, which has shown promising results in animal studies, causes the immune system to recognize and attack the highly addictive opioid before it reaches the brain.
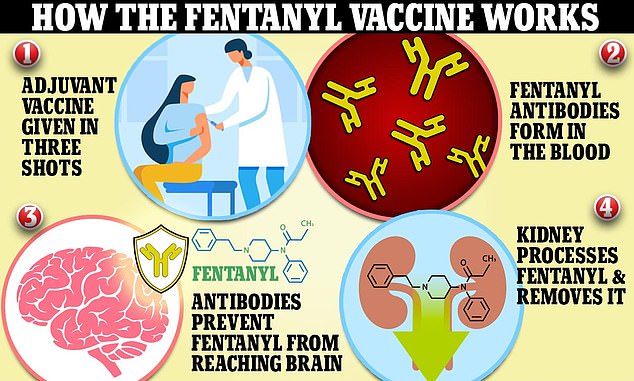
This prevents the drug from binding to receptors responsible for pain relief and euphoria. It is also said to prevent overdose, which occurs when the drug binds to receptors and disrupts breathing.
Researchers at the University of Houston who developed the vaccine plan to begin human trials in April 2025.
They hope the groundbreaking treatment can help curb the fentanyl epidemic, which kills more than 103,000 Americans each year.
Researchers have developed a three-shot vaccine that leads to the formation of fentanyl antibodies in a person’s bloodstream. These antibodies can block the drug from reaching the brain and destroy it completely. This in turn stops overdoses

A sad scene continues to play out on Kensington Ave in Philadelphia during Christmas last year. Fentanyl has caused a riot in the American drug supply, leading to a rise in overdose deaths.
If the vaccine is successful, it will be tested further, but researchers say it could take five to 10 years before it reaches hospitals.
It is estimated that a total of 103,451 people die from drug overdoses each year in the United States, with fentanyl being the cause of 70 percent of these deaths.
The drug is deadly in small doses – as little as three milligrams – and is 50 times stronger than heroin and 100 times stronger than morphine.
Dr. Colin Haile, an associate professor at the University of Houston who oversaw the vaccine’s development, said the team moved quickly to test the vaccine and get it into hospitals.
“We need to get something out there as quickly as possible to address this terrible problem,” he said. “The ultimate goal is to protect people and save lives.”
Doctors already use naloxone (brand name Narcan) to treat overdose patients, but the drug must be administered quickly to save patients.
The new vaccine would be offered to drug addicts, the researchers suggest, and would hopefully provide protection for a significant period of time. In fact, tests on rats have shown that the vaccine provides protection for at least ten weeks.
Experts have struggled to develop a vaccine against fentanyl since the 1970s because, unlike bacteria or viruses, opioids are not immediately recognized as invaders by our immune systems.
But they have found a possible approach: attaching part of the drug to a harmless part of a bacterium that triggers an immune response.
For their vaccine, the researchers linked some fentanyl to an enterotoxin, a chemical produced by the E. coli bacterium. This substance has already been tested in 15 human trials and caused virtually no side effects.
Its effectiveness has already been demonstrated in rat trials, unveiled in November 2022, which activated anti-fentanyl antibodies.
The chart above shows the number of Americans who die each week from drug overdoses. Nearly 2,000 people still die each week from medical emergencies.
The map above shows the change in drug overdose deaths by year. While the numbers are dropping in many states, they are still much higher than they were even five years ago.
In the Phase I study, the vaccine is administered to a small number of healthy people who are also addicted to opioids.
This is done to test whether the drug is safe for use in humans, to check for side effects, and to determine an optimal dosage.
It is not clear how many shots participants will receive, but in rat studies, the rodents were given three doses every three weeks. The price of a dose is not yet clear.
Researchers say the trials will likely take place in Australia or the Netherlands, although options in the US are also being considered.
But they expect challenges during the study because the population it will be tested on – drug users – can be difficult to track.
Dr. Jay Evans, director of the Center for Translational Medicine at the University of Montana, who is also involved in the research, said: ‘Compared to a normal clinical trial for infectious diseases, this is going to be more difficult.
‘The FDA is adamant that this vaccine should not be tested on healthy individuals who are not already addicted to opioids.
‘So we have to focus on patients in Phase I who have a history of opioid addiction, which is a more difficult population to recruit.
“It will take longer; the patient population will suffer more from side effects because they are drug users and they will be harder to trace.”
Researchers say the shot does prevent people from getting “high” on fentanyl, but it does nothing to prevent cravings and withdrawal symptoms or motivate someone to seek medical help.
People who use drugs will likely still experience a high, but it will be less intense because the fentanyl effect has been removed.
It is also not thought to interfere with other medications, such as morphine, meaning people with a medical emergency can still be treated.
The vaccine was purchased in November 2023 by start-up Ovax, which has since raised $10 million to fund further research.
Results from animal studies showed that antibody levels to the drug increased significantly between weeks four and six. Then, consistent protection was observed from the fourth through the tenth and final week of the study.
Data shows that fentanyl is toxic even in small doses. Overdose can cause a person to stop breathing and deprive the brain of oxygen, which can lead to death or permanent disability.