Ecstasy from your doctor in six months? Makers of MDMA drug for trauma patients to ask FDA to approve psychedelic treatment
- In the latest study, 71% went into remission after MDMA-assisted therapy
- It is used to treat PTSD, which affects 12 million American adults
- READ MORE: Patients tell DailyMail.com they’ve been healed by psychedelics
MDMA is on the verge of approval in the US after showing promise in final trial results as a powerful treatment for post-traumatic stress disorder (PTSD).
Researchers behind the groundbreaking studies expect to file a new application within months for the drug, which is popular in rave culture to dance the night away and feel more connected to the music and other ravers.
In the latest study of 53 patients with moderate to severe PTSD, 71 percent went into remission after taking the drug along with therapy.
The US Food and Drug Administration (FDA) may make a decision on approving the drug for the treatment of PTSD, if combined with talk therapy, as early as six months later.
The move could bring MDMA to some of the 12 million American adults who suffer from the debilitating condition.
Patients take a dose of the drug under supervision. Sessions with a therapist then help people process their trauma

Clinical trial participants received 120 mg or 180 mg of MDMA – the standard amount for one or two pills
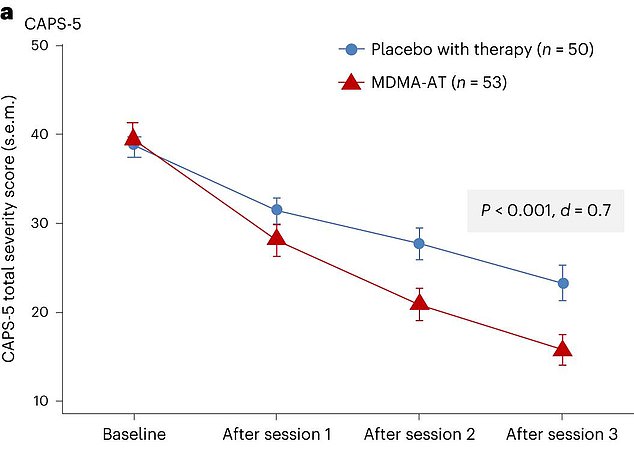
The CAPS-5 total score on the y-axis shows the average severity of patients’ PTSD symptoms
Also known as ecstasy or molly, MDMA has become part of a new frontier of psychedelics being repurposed as drugs for trauma and depression, along with ketamine, magic mushrooms and LSD.
If approved, MDMA-assisted therapy would be the first new treatment for PTSD in more than two decades.
It has been an illegal substance since 1985, when the Drug Enforcement Administration categorized it as a Schedule 1 drug – meaning the agency ruled it had no medical use and a high potential for abuse.
It is believed that before this, hundreds of therapists offered the drug to patients in North America and Europe to address trauma.
The Multidisciplinary Association for Psychedelic Studies Public Benefit Corporation (MAPS PBC), a company that develops prescription psychedelics, conducted the trials and has been advocating for the legalization of MDMA-assisted therapy since 1986.
In 2017, the FDA granted status from ‘breakthrough therapy’ to MDMA-assisted therapy as a PTSD treatment, which allowed its development to be accelerated.
A total of 104 patients diagnosed with moderate to severe PTSD took part in the latest study. On average, they had been living with PTSD for sixteen years.
The study group included people such as victims of childhood trauma, combat veterans and survivors of sexual assault.
Many of them also suffered from depression and alcohol addiction.
Participants worked with two therapists and had three 90-minute preliminary talk therapy sessions, followed by three treatment cycles one month apart.
The treatment cycle included an eight-hour experimental session in which the patient took MDMA or a placebo in addition to talk therapy, and then received three more 90-minute talk therapy sessions.
About 53 patients received MDMA, and 51 received an inactive placebo.
The study was double-blind, meaning that neither the patients nor the therapists knew who had taken what.
By the end, 87 percent of people who used MDMA had reduced symptoms, the researchers said. And about 71 percent of them improved so much that they no longer met criteria for PTSD.
In the placebo group, 69 percent had less intense symptoms and 48 percent no longer met diagnostic criteria.
The research was published yesterday in the journal Nature.
Critics of the study have said the result may not be strong enough to gain FDA approval.
Dr. Allen Frances, professor emeritus of psychiatry at Duke University, told the New York Times.
“The benefits in the active group were actually not much greater than the benefits in the placebo group,” Dr. Allen Frances, professor emeritus of psychiatry at Duke University, at the New York Times.
‘MDMA treatment would add enormous costs to the treatment system while providing only a small, specific benefit – thus resulting in a massive misallocation of already very scarce resources.’
