Childhood asthma drug linked to dozens of suicides and terrifying hallucinations of ‘red-eyed demons’ is STILL prescribed to 12 million Americans every year – despite FDA warnings in 2020
Parents are not warned about the dangers of an asthma drug linked to dozens of suicides, hallucinations and self-harm.
The Food and Drug Administration (FDA) added a black box warning to the label of Singulair, the generic name montelukast, in March 2020 after decades of reports of mental health problems in patients.
The black box warning was primarily intended for doctors who prescribed the drug and who had to pass it on to patients.
But almost two years later, data shows that the number of prescriptions for the drug has barely changed – with 12 million prescriptions in 2022, of which almost 1.6 million are for children.
Campaigners are warning the drug may be the cause of an unrecognized ‘mental health crisis’, with patients previously saying they saw demons with red eyes, walls melting and hearing voices. They urge parents and doctors to ensure they are aware of the risks.
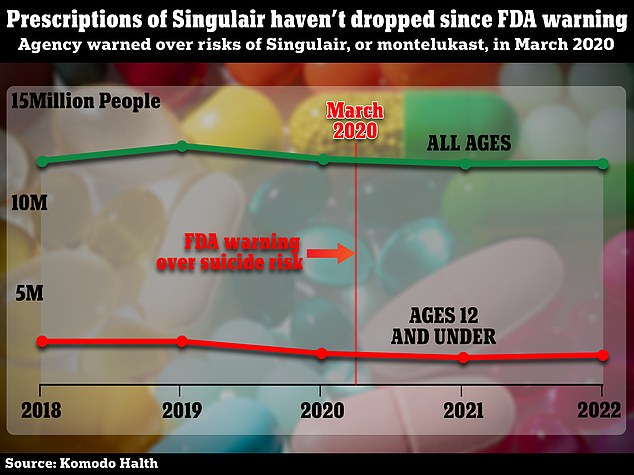
The chart above shows how prescriptions for Singulair have barely budged since the FDA’s warning


The FDA added the above warning to the first page of the drug’s package insert in March 2020, but experts fear it has gone largely unnoticed
Singulair – made by pharmacy giant Merck – became hugely popular in the early 2000s as a treatment for allergies and severe asthma, especially in young children.
It works by preventing the release of a chemical in the airways, which can cause them to swell and produce a lot of mucus, putting them at risk of becoming blocked.
The drug’s enormous popularity was related to the fact that it appeared to be a safe alternative to corticosteroid inhalers, which are more effective but more cumbersome to carry. It even came in a kid-friendly, cherry-flavored chewable pill.
But officials began raising alarms about possible links between the drug and mental health problems.
Data from healthcare analytics company Komodo Health – obtained by the New York Times – shows that the recipes have hardly changed.
Overall, this figure has not changed: in both 2019 and 2022, twelve million people filled at least one prescription.
There has been a decline in children – with almost 1.6 million prescriptions last year compared to 2 million in 2020 – but this is still a high number.
Komodo Health’s data is based on claims to Medicaid and Medicare, as well as claims submitted to private insurers.
Some experts fear that many doctors are unaware of the warnings about the drug, which were announced on the FDA’s website.
Dr. Reshma Ramachandran, a family physician at Yale University in Connecticut, said many doctors had never heard of the concerns.
She urged the FDA to do “much more in terms of communicating directly with physicians and through more active channels.”
She suggested the agency could impose mandatory retraining of doctors to make them aware of the risks.
The FDA has already sent an email alert, communicated the alert to medical groups, and published an article on the issue in a medical journal.
Pharmacists are also required to hand out a medication guide with the warning to patients – although experts fear it often goes unread.
A number of doctors who have tried to switch patients to other drugs, such as zafirlukast, also report patient resistance and demands to be allowed to use Singulair again.


Singulair became popular in the early 2000s for its ability to treat allergies and asthma with just a chewable tablet, especially for children




Virginia native Nicholas England fatally shot himself in the head at the age of 22, just weeks after taking the generic version of Singulair. At right, Genevieve Bracken, 14, of Utah, died by suicide days before Christmas after switching to an adult dose of the drug
In the black box warning on the drug’s label, the FDA says the drug can cause “serious neuropsychiatric events.”
This is thought to be because the drug can cross the blood-brain barrier, as shown in studies in rats, and then wreak havoc with neurotransmitters.
A study by the FDA, which analyzed data from 1998 to 2018, found that the drug was linked to as many as 82 suicides – 19 of which were in children.
The agency has also received thousands of reports of side effects — including depression — since people started taking the drug.
Warnings were first added to the drug in 2008, when the manufacturer was forced to add a patient with psychiatric risk to the label, before the 2020 warning was added.
Those affected by the drug’s mental health side effects include 22-year-old Nicholas England.
The youngster shot himself in the head in 2017, just two weeks after starting a generic version of the drug – with his parents saying he had no previous mental health problems.
Others affected include the six-year-old son of Nicole Sims of Tennessee, with the mother saying her son began having hallucinations and imagining a woman at the window while he was taking the drug.
His drawings were also turned into those of red-eyed demons – only turning back into a smiling boy fishing under the sun after he came off the medication.
Genevieve Bracken, a 14-year-old from Utah, died by suicide while taking the drug.
She had been taking the drug since she was seven years old, but became more depressed when she switched to an adult dose at age 13.
Shortly before her Christmas suicidal days, her mother said she attributed her obsessive hand-washing and moodiness to the Covid pandemic.
It’s not clear how often the drug causes mental health problems and suicides, and Merck has yet to be asked by the FDA to investigate.
