Americans left BLIND, paralyzed and with teeth falling out after Ozempic – now they are suing for millions
When Monica Church was prescribed Ozempic to treat her diabetes in October 2023, she was told it would help relieve her symptoms.
Near Christmas, the 55-year-old Michigan woman was hospitalized for two weeks due to abdominal pain, vomiting and stomach paralysis.
She lost the ability to eat foods she used to enjoy, such as pizza and bread, and now only eats small meals to avoid upsetting her stomach. She said her doctor did not tell her about these side effects.
Ms. Church is not alone. She is one of a growing number of people joining lawsuits against Novo Nordisk and its parent company, Eli Lilly, over claims that they failed to adequately depict certain risks of the drug on the packaging.
Robert King, a New York-based attorney who represents 400 different patients, told DailyMail.com that stomach problems are by far the most common complaint.
But he also represents people who suffered blood clots after starting the drug, and people who claim the drug caused blindness, caused their teeth to fall out and left them paralyzed.
He said people repeatedly tell him they wanted to be better informed about the risks of the drugs before taking them.
Mr King said: ‘I don’t think anyone would agree with possible blindness. You know, that’s a major life event that no one would ever suspect is related to a weight loss problem.”
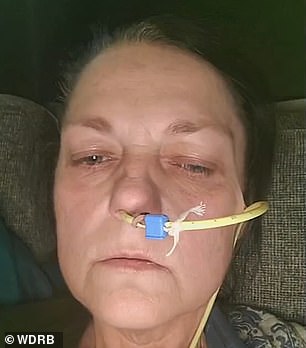
Jacqueline Barber from Louisville, Kentucky slept on the couch with a trash can next to her for about a year. She lost 140 pounds, her teeth started to crumble due to the stomach acid in her teeth and she became so weak that she had to use a walker to get around
Ozempic users have previously told DailyMail.com that the drug has stopped them craving alcohol
Ozempic and other drugs such as Mounjaro, Wegovy, and Trulicity are in a class of drugs called a GLP-1 agonist.
They mimic a hormone that controls the feeling of fullness and how quickly food moves through the body.
These medications were originally created to treat diabetes, but noticing how well it helped people shed pounds, doctors began prescribing them to people trying to lose weight.
A May 2024 poll found that one in eight adults uses a GLP-1 agonist – which could represent as many as 30 million people.
Mr. King said these lawsuits began in the fall of 2023, but over the past year the number of people joining suits has steadily increased.
Cecily King, 43, Kentuckian has been taking GLP medications since September 2021.
Over time, she developed gastroparesis, a paralysis of the stomach that can cause vomiting, constipation, malnutrition, acid reflux and blood sugar problems.
In Ms. King’s case, holding her stomach caused severe, persistent vomiting, diarrhea and abdominal pain, sending her to the emergency room.
This damage is permanent and she still suffers from gastrointestinal problems.
Mrs. Church, the patient from Michigan, told USA Today that living with this symptom was difficult.
She said, ‘I couldn’t eat anything. I couldn’t drink anything. I had such a burning feeling in my stomach and chest that nothing helped.’
Ms. King is among the plaintiffs suing Novo Nordisk and Eli Lilly. Her lawsuit alleges that the companies: “downplayed the severity of the gastrointestinal events caused by the GLP-1RAs.”
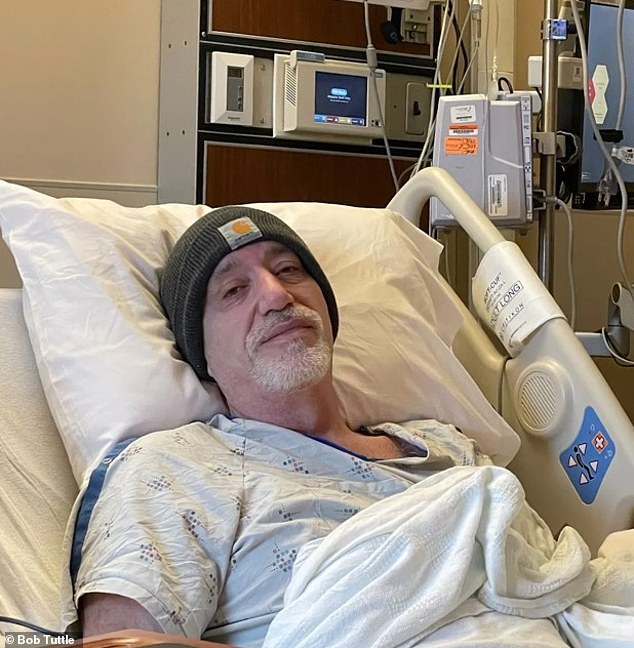
Bob Tuttle of Tennessee became so ill from gastric paralysis that he had to quit his job as a safety consultant on an oil rig

Mr Tuttle had to be flown off the platform in the Gulf of Mexico after being unable to keep food down for four days. Within a week he was diagnosed with gastric paralysis
Gastroparesis can become so severe that it threatens your life, as in the case of Louisville’s Jacqueline Barber, 49. Barber told Time that her doctor said it would “do wonders” for her diabetes.
Instead, she said it destroyed her stomach, leaving her bedridden and vomiting constantly for the year she took the drug.
She lost 140 pounds, her teeth started to crumble from the stomach acid, and she had to start using a walker because she became so weak.
Mr King said there are also some cases where patients claim life-threatening blood clots are linked to their GLP-1 drug.
Florida man Roderick Shirely, 83 started taking Ozempic in 2022 on the advice of his endocrinologist. Initially he tolerated it well.
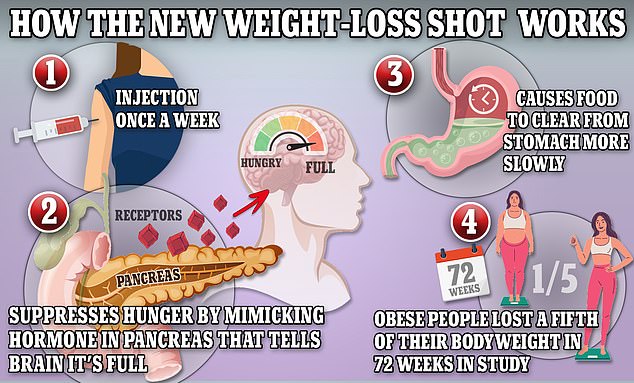
Ozempic and its sister drug Wegovy work by prompting the body to bind to a receptor called glucagon-like peptide-1 (GLP-1), a protein that activates hormones in the brain that keep the stomach full and tell the body to stop eating. and avoid cravings
But in September 2023, Mr Shirley started having chest pains and went to the doctor. There they found a large blood clot in his hip and lungs, a so-called deep vein thrombosis. broken down and traveled to his lungs. This potentially cut off and damaged the blood supply to the life-saving organ.
He required emergency surgery and almost died twice while on the operating table. He also had three cardiac arrests.
He was then hospitalized for three weeks, where he destroyed medical bills. Mr. Shirely is another plaintiff in the case, and his suit claims that his: “life has been forever changed because of his use of Ozempic.”
There is one study conducted by scientists at Chenzhen Longhua District Central Hospital in China, linking GLP drugs to the type of blood clots suffered by Mr Shirely.
However, the evidence is inconclusive. There are also study which suggest the drug may reduce blood clots and related heart problems.

Dana Filmore of Columbus, Ohio, now lives on a diet of protein shakes and Jell-O. Her gastric paralysis related to Ozempic prevents her from eating solid food
The companies that produce these drugs vehemently deny these claims.
A spokesperson for Novo Nordisk previously told DailyMail.com that these lawsuits are without merit. They said the side effects and risks of Ozempic are clearly stated on the label.
They added: ‘Novo Nordisk stands behind the safety and efficacy of all of our GLP-1 medicines when used as indicated and when taken under the care of a licensed healthcare provider.’
Ozempic’s FDA-approved warning label lists “gastrointestinal side effects.”
It appears that no label mentions gastroparesis.
Yet the plaintiffs in these cases argue that their goal is not to get rid of Ozempic, but to help people become more aware of the risks of using it.
Barber said, “I just want people to get the education that I didn’t have.”
