America gets a new Alzheimer’s drug in its arsenal: FDA approves Eli Lilly treatment that slows symptoms
The US FDA has approved donanemab, a treatment for Alzheimer’s disease designed to slow the progression of the early signs of dementia.
Donanemab, developed by Indianapolis-based Eli Lilly, is a monoclonal antibody treatment that helps the body clear the characteristic plaque that builds up in the brain and causes dementia.
Clinical studies have shown that the treatment can slow cognitive decline in patients by up to 35 percent.
Now approved, Eli Lilly said it will be sold under the name Kisunla and administered through monthly IV infusions. It will cost $695 per vial — about $32,000 a year.
While donanemab is a breakthrough in the treatment of Alzheimer’s, experts have raised concerns about the known risks of brain hemorrhages, which have killed several trial patients.
Donanemab, developed by Indianapolis-based Eli Lilly, is a monoclonal antibody treatment designed to slow the progression of early signs of Alzheimer’s disease
Donanemab will be sold under the name Kisunla and will cost $695 per vial, which equates to about $32,000 per year
Dr. Howard Fillit, Co-Founder and Chief Scientific Officer at the Alzheimer’s Drug Discovery Foundation (ADDF), said in a press release: ‘This approval marks another step forward in developing the standard of care for people with Alzheimer’s disease, which will eventually include an arsenal of new treatments and provide much-needed hope to the Alzheimer’s community.
‘If Alzheimer’s is diagnosed and treated earlier, the progression of the disease can be significantly slowed down. This gives patients more time to remain independent for longer.’
According to Eli Lilly, the drug slowed cognitive and functional decline in patients by 35 percent compared to placebo over 18 months.
It also reduced the risk of patients developing advanced Alzheimer’s by 39 percent.
The study also found that donanemab reduced the amount of amyloid plaques in participants’ brains by an average of 61 percent after six months, 80 percent after 12 months, and 84 percent after 18 months, compared with placebo.
Although donanemab was touted as a potential therapy, a quarter of patients in Eli Lilly’s trial experienced brain swelling and three people died from brain swelling or bleeding attributed to the drug.
In addition, patients who received donanemab had a slightly higher death rate: two percent, compared to 1.7 percent who received placebo.
As the U.S. aging population continues to grow, so will the number of dementia cases. Currently, an estimated 6.7 million Americans have Alzheimer’s disease — the most common cause of dementia — the vast majority of whom are over the age of 65.
This number is expected to rise to almost 13 million by 2050.
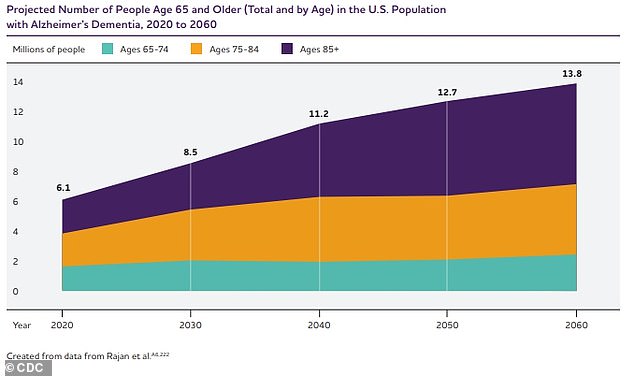
The graph above shows the estimated projection of the number of patients with Alzheimer’s disease in the US until 2060.
Although the root cause of Alzheimer’s disease is still debated, scientists believe the damage likely results from an abnormal buildup of proteins (amyloid and tau) in and around brain cells.
In Alzheimer’s patients, amyloid proteins are not effectively removed from the body and eventually form plaques in the brain. Tau proteins detach from neurons and form tangles.
Both can lead to the death of neurons, making it difficult for signals to travel through the brain.
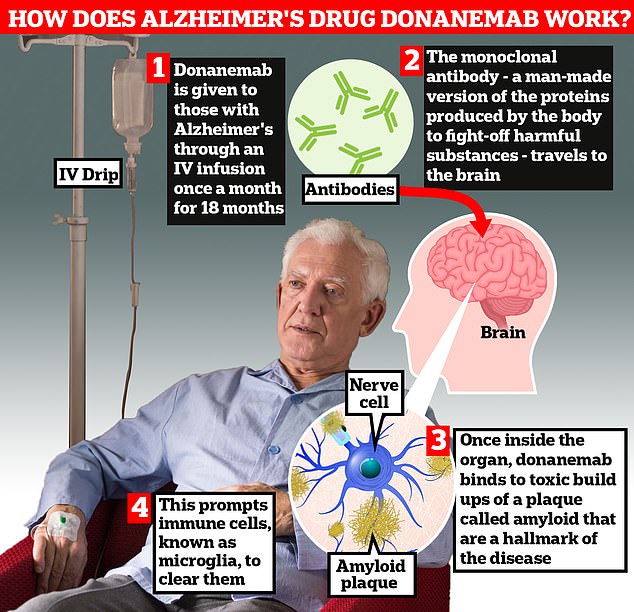
Donanemab is designed to target amyloids. The monoclonal antibodies are injected into the body via IV and travel to the brain.
Once in the organ, donanemab binds to toxic deposits of amyloid plaque, which causes immune cells called microglia to clear the bacteria.
Patients stop taking donanemab once the amyloid buildup has cleared from their brain.
The treatment is intended for people with early symptoms of Alzheimer’s disease or mild cognitive impairment.
Donanemab is not the first monoclonal antibody to be approved by the FDA. Lecanemab, sold as Leqembi, is already used to treat Alzheimer’s patients.
Despite the risks, patients and families taking part in the donanemab trial said the new dementia drug finally offers patients a glimmer of “hope.”
Jim Sirois, now 68, was diagnosed with early-onset Alzheimer’s in 2020. He had difficulty speaking and had become so forgetful that he could not remember where he had been the day before.
Mr Sirois was enrolled in the drug arm of the clinical trial, which his wife told DailyMail.com in August 2023 had effectively ground to a halt over the past year.
Sue Sirois, 65, said: ‘I compare Jim’s decline to that of a friend I know who was diagnosed with Alzheimer’s at the age of 56. She recently died and only survived for six years, whereas Jim has now been ill for over three years and is still pretty much the same as before.’
Ms Sirois added: ‘I would say that those who have the opportunity to get this drug should go ahead and get it, because what else do you have? What other hope do you have? This drug is the only hope that people have now – there is no other hope for the disease.’
