The superbugs lurking in your city: Microbes resistant to disinfectants are breeding in offices, schools and malls, study warns
Since the Covid-19 pandemic, hand sanitizers and sprays have become a staple in cities around the world.
But according to a new study, our attempts to create “sterile urban environments” in offices, schools and shopping centers could be backfiring.
Instead, we may be creating an army of disinfectant superbugs.
Researchers from Xi’an Jiaotong-Liverpool University in China took swabs from indoor surfaces around Hong Kong, as well as from human skin.
They discovered that microbes – tiny living things that are too small to see without a microscope – consume chemicals in cleaning products to survive.
Although the study focused on samples from Hong Kong, the team believes that similar findings could be observed in other cities as well.
The study comes shortly after scientists from Darwin Bioprospecting Excellence SL discovered radiation-resistant microbes that reproduce in microwaves.
Researchers discovered that microbes – tiny living things that are too small to see without a microscope – consume chemicals in cleaning products to survive. Clockwise from top left: Streptococcus, mixed-species microbial biofilm from the human body, Bacillus and Malassezia lopophilis
For the study, published in Microbiome, the researchers collected 738 samples from various environments including subways, homes, public facilities, piers and human skin in Hong Kong.
Lead author Dr.
‘Examples of built environments include residential buildings, offices and public transport systems such as subways or subway stations,’ Dr Tong told MailOnline.
‘Our use of cleaning and other manufactured products creates a unique setting that places selective pressure on microbes to which they must adapt or be eliminated.’
To understand how they have adapted to urban conditions, the team analyzed the genomic content of the microbes in the laboratory using a method called ‘shotgun metagenomic sequencing’.
‘Shotgun metagenomic sequencing involves extracting genomic DNA from microbes and sequencing them to analyze their genetic material at the gene level,’ Dr Tong told MailOnline.
‘Each gene codes for a specific function, allowing us to understand the strategies microbes use to adapt and survive in the built environment.’
In total, the research team identified 363 previously unidentified microbial strains that live on our skin and in the environment around us.

With the addition of hand sanitisers and sprays, indoor spaces have hardly been the same since the Covid pandemic. In the photo a shopping center in Hong Kong
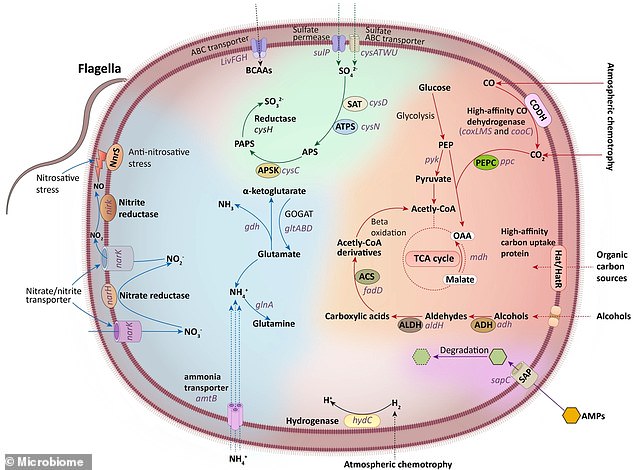
This figure summarizes the basic metabolic pathways of Eremiobacterota, including carbon, nitrogen, and sulfur metabolism, based on the functions encoded by its genes.
Some of these strains carried genes for metabolizing chemicals in common cleaning products, such as alcohol and ammonium ions, and used them as carbon and energy sources.
This includes a strain of Candidatus Eremiobacterota – a bacterium previously found only in Antarctic desert soil.
‘We have found a new microbial strain of Eremiobacterota, well adapted to residential areas and human skin, that can metabolize alcohol – a key ingredient in many hand sanitisers,’ Dr Tong told MailOnline.
‘This species can use alcohol as a carbon source, which then contributes to carbon metabolism for energy production.
‘In addition, the strain can metabolize inorganic salts, such as nitrate, sulfate and ammonium, which may be present in some cleaning agents.’
None of the microbes identified in the study were found to be pathogenic, that is, capable of causing disease in humans.
However, the team discovered a new strain in the genus Pseudomonas that carries a large number of genes associated with antimicrobial resistance (AMR).
AMR is when bacteria and other microbes adapt and evolve in response to modern chemicals designed to kill them, turning them into ultra-strong “superbugs.”

Pictured is Micrococcus luteus, a bacterium that is generally non-pathogenic but can cause opportunistic infections in people with weakened immune systems (file photo)
This ongoing health crisis could turn everyday injuries and routine surgeries into life-and-death events, undoing decades of medical advances and potentially causing millions of deaths every year.
The team also identified eleven previously uncharacterized strains of Micrococcus luteus, a bacterium that is generally non-pathogenic but can cause opportunistic infections in people with weakened immune systems.
‘Prolonged exposure to sub-optimal concentrations of disinfectants could stimulate microbial evolution, causing some microbes to develop resistance and even use disinfectants as an energy source,’ Dr Tong told MailOnline.
‘However, the specific microbes present may vary depending on the local environment.’
The team is now investigating pathogenic microbes in intensive care units, which are exposed to ‘rigorous and extensive’ disinfection routines.
‘While cleaning products can effectively neutralize or eliminate most harmful microbes, the disinfectants we use in everyday life are often applied less frequently and are milder than those used in clinical settings,’ added Dr Tong.
‘As a result, some microbes may adapt and develop increased resistance to these milder disinfectants, allowing them to survive over time.
‘However, additional experimental evidence is needed to confirm this idea.’
