Top doctors warn against $1,000 blood tests that claim to detect 50 types of cancer – and urge Americans not to skip critical screenings
Top doctors have warned against tests that claim to detect dozens of types of cancer, urging Americans that “we don’t have the evidence” and should stick to regular screenings.
Multicancer detection tests (MCDs) have emerged as potential alternatives to invasive tests such as colonoscopies and mammograms. These tools, which consist mainly of blood draws, are intended to detect abnormal proteins and other cells consistent with cancer.
Similar to full-body MRI scans promoted by Kim Kardashian and Gwyneth Paltrow, many MCDs also claim to contract the disease before symptoms begin, meaning cancer is detected at earlier stages.
However, researchers presenting at the American Society of Clinical Oncology meeting in Chicago this weekend said that while early studies are compelling, they would not recommend them to their patients and that they are “not quite ready yet” to be used on a large scale are prescribed.
They also warned that MCDs are at risk of false positives, which could lead to patients undergoing a series of unnecessary tests.
And these tools are not covered by insurance, as just one test costs more than $1,000.
The Guardant’s Shield blood test claims to detect colorectal cancers. Although the FDA considers it safe, agency researchers say more research needs to be done on MCDs
The warnings come as health officials lower screening age guidelines for colorectal cancer and breast cancer to stem a surge in young people with the disease.
Dr. Chyke Doubeni, a family medicine physician at Ohio State University, said in a presentation Friday, “We don’t have the evidence. I don’t think these tests are ready for primetime at the moment.”
“The risk of potential harm is there no matter how you look at it.”
Several recent reports on MCDs have shown promise, including two studies from the University of Oxford. In the first study, the team compared how proteins in blood samples from Britons diagnosed with cancer differed from those from those who did not have the disease.
They found 182 different proteins on average three years before the cancer diagnosis.
In the second study, 40 proteins in the blood were found to influence a person’s risk of developing nine different types of cancer: bladder, breast, endometrial, head and neck, lung, ovarian, pancreatic, renal and malignant non-melanoma.
Furthermore, research published earlier this year found that the Galleri test, which claims to detect 50 types of cancer and is being tested in Britain by the NHS, detected 18 types of early-stage cancer and claimed to be 93 percent accurate .
However, Paul Pharoah, a professor of cancer epidemiology at Cedars-Sinai Medical Center, said at the time that the “holy grail” of early detection was still far away because more in-depth research is needed.
‘Many of these types of tests have been developed or are in development. This paper reports on the first results of the development of such a test,” he said. ‘Although the results are promising, it is far too early to be confident that this test will prove useful for the early detection of cancer.’
Dr. Robert A Smith, oncology researcher at the American Cancer Society, said in the presentation that a potential benefit of MCDs is that they can detect cancers at an earlier stage.
“It would mean that the disease is detected earlier in its natural history than the symptoms, which suggests that the prognosis would be better,” he said.
However, he also noted that these “should not replace regular screenings.
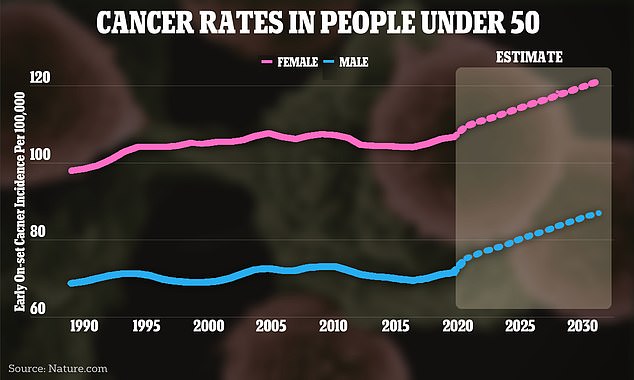
The above graph shows the change in cancer rates around the world
MCDs also carry the risk of false-positive results. “Although the false positive rate is low, the intended audience is hundreds of millions of people,” Dr Brewer said.
This could lead to patients having to undergo a series of unnecessary, expensive and invasive tests, such as biopsies, for a negative diagnosis.
Dr. Ernest Hawk, vice president, Cancer Prevention and Population Sciences at MD Anderson Cancer Center in Texas, said at a hospital blog post: ‘Any positive blood test requires subsequent diagnostic evaluation.’
‘Endoscopy, radiological imaging and biopsies all take additional time, increase costs and can result in additional risks due to the procedures themselves.
“We need to ensure that we reduce cancer incidence or improve cancer-specific survival with this cancer screening prevention test,” Dr Brewer said.
Dr. Brewer even suggested that the FDA and other agencies could more closely monitor and place restrictions on the tests before they hit shelves.
Earlier this month, the agency showed support for the Guardant Health Shield Test, which is designed to test for colorectal cancer, with the majority considering it safe.
However, FDA committee member Dr. Charity J Morgan said that while the test was ‘better than nothing for patients who get nothing’, it is ‘no better than a colonoscopy.’
Dr. Jonathan M Marron, a pediatric oncologist at Boston Children’s Hospital, said in the presentation that the research so far is moving in a positive direction, and that along with caution, optimism is key.
“MCDs may have value now and in the future, but the value has yet to be clearly demonstrated.”
‘There remains a lot of uncertainty.’
