Ozempic maker’s new slimming pill, which is TWICE as good as a blockbuster skinny jab, won’t be available in Britain and the US until 2026
A new, successful weight loss pill, made by the maker of Ozempic, could be ready as soon as 2026.
Pharmaceutical titan Novo Nordisk yesterday revealed promising early results for its experimental drug amycretin, showing patients were able to lose more than 13 percent of their weight after just twelve weeks.
In contrast, the groundbreaking weight loss drug Wegovy has been proven to help people lose up to six percent in the same period.
A phase two drug trial will begin in the second half of the year with results expected in early 2026, the company said.
It means the treatment – which is subject to even larger trials – could be available to consumers soon afterwards, pending the final regulatory hurdles.
Pharmaceutical titan Novo Nordisk yesterday revealed promising early results for its experimental drug amycretin, showing patients were able to lose more than 13 percent of their weight after just twelve weeks. In contrast, Wegovy has been proven to help people lose up to six percent in the same period.
Amycretin is similar to Wegovy and sister drug Ozempic.
It targets the same glucagon-like peptide-1 hormone, which regulates appetite and the feeling of fullness.
In addition, it stimulates another agent called amylin, which also reduces hunger and slows stomach emptying.
The results showed that the drug also appeared safe and well tolerated in the 16 people who took it for three months, and whose average weight at the start of the trial was 14st (196 pounds).
The speed at which people lost pounds with amycretin far exceeded the time it took Wegovy and Ozempic patients to lose that amount in studies – 68 weeks.
The side effects experienced were also consistent with those of the other GLP-1 drugs, Novo Nordisk said.
These usually include gastrointestinal problems such as nausea, vomiting, constipation and diarrhea.
Martin Holst Lange, head of development at Novo Nordisk, said he expected the pill could be available to consumers “within this decade.”
“I never commit to timelines, but I would be very happy to say at least within this decade,” he added.
Meanwhile, the Medicines and Healthcare products Regulatory Agency (MHRA), which oversees the safety of medicines used in Britain, said it was aware of the study results.
Julian Beach, interim executive director of healthcare quality and access at the MHRA, told MailOnline today: ‘We are aware of the clinical trial results shared by Novo Nordisk for the weight loss drug amycretin.
“We will monitor the results and assess each submission against the indications outlined in the findings when submitted to us.”
Katharine Jenner, director of The Obesity Health Alliance, said the study results “give great hope to those who cannot control their weight due to many complex factors.”
She told MailOnline: ‘Obesity is a chronic, relapsing condition with many causes. Medicines alone will not be the answer to Britain’s extremely high obesity rate.
‘We need to take action to ensure that as few people as possible reach the stage where they need these types of medicines.
‘It is vital that we tackle the root causes of obesity, such as the flood of unhealthy food and drink that is constantly marketed and promoted to us, so that we fail to treat people and then send them back to the conditions in which they became ill . ‘
Yesterday, Novo Nordisk CEO Lars Fruergaard Jorgensen said the obesity market is “a huge catwalk.”
Analysts predict it could be worth £70 billion ($90 billion) in the coming years.
The results moved Novo Nordisk into the 12th most valuable company rankings, with a valuation of £440 billion ($566 billion), surpassing those of Tesla and Visa.
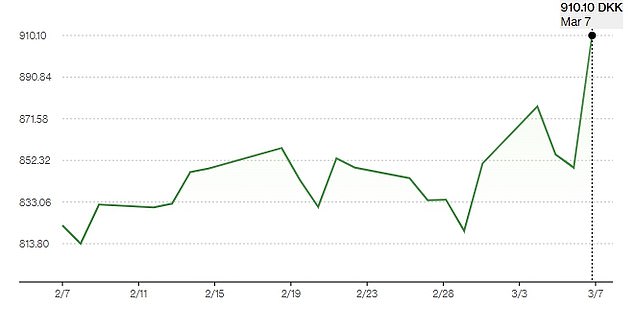
Shares of Novo Nordisk rose 8 percent in Copenhagen on Thursday to hit a record high after revealing early results in a trial of a pill-form anti-obesity drug
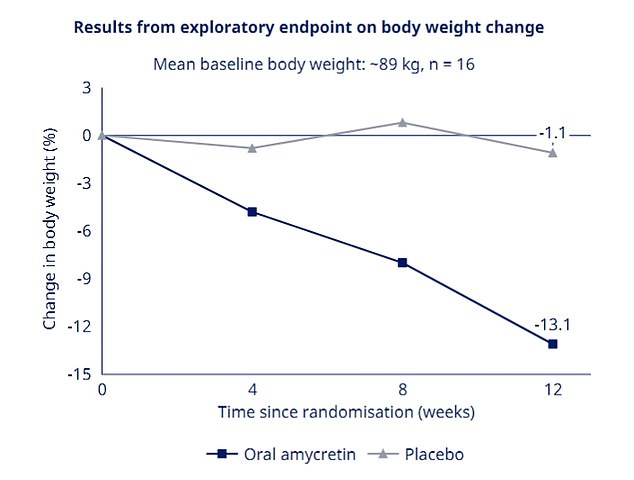
Trial participants lost more than 13 percent of their body weight in just three months, a rate that far exceeds the time it takes for Wegovy to work
Novo Nordisk also said it would continue working on another treatment, CagriSema.
It is a combination therapy that contains semaglutide to target GLP-1, as well as a drug called cagrilintide, an analogue of the hunger-reducing substance amylin.
It comes as health experts last month urged Britons not to rely on weight loss jabs as a quick fix to resolve the crisis in the same Lords meeting, warning they are ‘not the answer’ and ‘inevitable in due course’ will cause problems’.
Henry Dimbleby, the government’s former food tsar, told the Food, Diet and Obesity Committee: ‘I fear that what will happen, which has all kinds of negative connotations, is that if action is not taken to improve the food system, we will increasingly help ourselves to the problem with medicines.
“We’re going to end up, just like you, with 30 percent of the population on antidepressants, with 30 percent of the population on diet suppressants, and you’re going to move profits from the food companies to the pharmaceutical companies.”
Ozempic is currently only available on the NHS as a treatment for controlling blood sugar levels in people with type 2 diabetes.
Because of its dramatic weight-loss effects, doctors and pharmacists have distributed it ‘off-label’ to people who want to lose weight.
However, health chiefs urged against this due to supply issues and warned it would put the lives of diabetics at risk.
Wegovy was approved earlier this year specifically for weight loss.
A one-month supply is available privately from Boots and Superdrug for around £200. The eligibility criteria for people wanting to get the drug on the NHS is strict.
A lack of exercise, combined with an unhealthy diet, is blamed for Britain’s growing obesity epidemic.
The latest NHS data shows that 26 percent of adults in England are obese and a further 38 percent are overweight but not obese.
