WARNING: Lifesaving Cancer Treatment Can CAUSE Cancer Itself, Says New FDA Safety Label
A life-saving cancer therapy could actually cause new cancers in patients who receive the treatment.
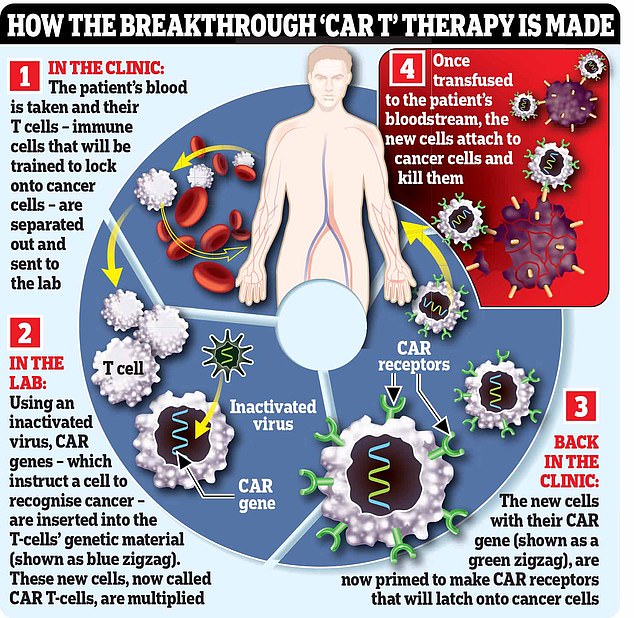
CAR-T therapies have saved tens of thousands of terminally ill blood cancer patients since 2017, by extracting immune cells from the body and redesigning them in a laboratory – before infusing them back into patients.
But in a letter issued this week, the Food and Drug Administration said the treatment should now carry a black box warning — the most serious safety warning — that they may cause cancer.
However, the agency emphasized that the benefits of the treatment “still outweigh the potential risks.”
An illustration of a T cell, blue, attacking a cancer cell, red. This underlines how CAR-T therapies work
CAR-T therapies, or chimeric antigen receptor T cell therapies, were first approved in November 2017 and are reserved for cancer patients who would otherwise die without this therapy
They are currently investigating 19 cases of cancer among patients, compared to the 27,000 who received the treatment – suggesting a cancer risk of around one in 1,500 patients.
Experts say the groundbreaking treatment can cause cancer by disrupting cell DNA, which then leads to other cancers.
It is not unheard of for cancer therapies themselves to cause the disease, even though this is already an established risk for radiation and chemotherapy.
CAR-T therapy, or chimeric antigen receptor T-cell therapies, involves collecting white blood cells from a patient and then genetically altering them in a laboratory to target cancer cells.
These are then injected back into a patient and get to work eliminating the cancer.
The treatment is around 76 percent effective against blood cancer and was first approved in 2017 – there are now six versions available.
In its update, the FDA said the black box should now include the following warning on the prescribing information: “T-cell malignancies have occurred following treatment with BCMA- and CD19-targeted genetically modified autologous T-cell immunotherapies, including KYMRIAH.”
T cells are a type of white blood cell utilized by therapy, with malignancies referring to when these cells become cancerous.
The letter was updated to the drugmakers: Bristol Myers Squibb, for Abecma and Breyanzi; Gilead Sciences’ Kite Pharma, for Yescarta and Tecartus; Carvykti from Johnson & Johnson; and Novartis, for Kymriah.
The companies said in statements that they agreed to add black box warnings to their drugs.
A Novartis spokeswoman told DailyMail.com in a statement: ‘Novartis places the highest priority on patient safety and is committed to fully understanding the effects of its medicines.
“Novartis will work with the FDA to appropriately update Kymriah prescribing information in the best interest of patients.”
She added: ‘Novartis has not yet found sufficient evidence to support the causal relationship between Kymriah and secondary T-cell malignancies and remains confident in the favorable benefit-risk profile of Kymriah.’
This is the third black box warning to be added to the drugs, with the others also including cytokine storms – an immune system overreaction that can be fatal – and neurological toxicities.
The cancer warning was already detailed on the prescribing information, but had not previously been included in the black box.
The National Cancer Institute estimates that there will be approximately 60,000 new cases of leukemia in 2023, resulting in more than 20,000 deaths.
A third of patients with blood cancer die within five years of diagnosis.
The Leukemia and Lymphoma Society estimates that 90,000 people will be diagnosed with lymphoma, a cancer of the lymphatic system that includes Hodgkin (HL) and Non-Hodgkin (NHL) lymphoma, and that an estimated 21,000 people will die from the disease. The five-year survival rate for HL is 96 percent and 85 percent for NHL.
Myeloma, another form of blood cancer, is expected to affect 35,000 people and cause 12,500 deaths. The five-year survival rate is 77 percent.
