Common anxiety pill taken by 16 million Americans is not very effective and its efficacy has been greatly exaggerated, Harvard analysis finds
It’s known to be addictive, harmful to the body, and risky for overdoses — and now scientists say Xanax may not even be that effective.
Researchers from Harvard University and Oregon Health & Science University re-evaluated studies and research that looked at the effectiveness of Xanax in treating panic disorder.
They found several examples of “publication bias” that exaggerated how positive the results were.
The researchers – including a former reviewer for the US Food and Drug Administration (FDA) – concluded that while the drug was still more effective than a placebo, publications boosted the drug’s efficacy by about 40 percent.
Xanax, also known as alprazolam, was approved by the FDA in 1981 and belongs to the class of benzodiazepines, a group of depressant medications used to relieve anxiety.
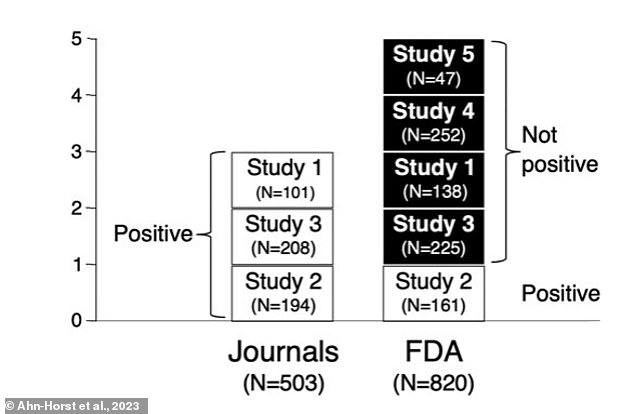
Of the five studies reviewed by researchers, three were presented as positive. However, when the FDA reviewed the studies, only one was considered positive
Publication bias is the inability to publish the results of a study based on the direction or strength of the study results.
It happens when the outcome of research influences the decision to publish findings. However, only publishing results that show a significant finding upsets the balance of the findings in favor of positive results.
Senior author Dr. Erick Turner, professor of psychiatry and former FDA reviewer said: ‘Our study throws some cold water on the efficacy of this drug. It shows that it may be less effective than people thought.”
The study analyzed published and unpublished data from five clinical trials reviewed by the FDA for Xanax for the treatment of panic disorder, an anxiety disorder in which you regularly experience sudden attacks of panic or anxiety.
Of the five studies reviewed, only three had been published and the researchers believed that only one had positive findings.
Likewise, when the FDA reviewed the studies, only one was deemed positive and the other four were classified as “fails on their face.”
Of the four non-positive studies, researchers found that two had been published that produced a positive result, and the other two had not been published at all.
The studies were conducted between 1986 and 1989 (not positive), 1988 and 1990 (positive), 1994 and 1995 (not positive) and two took place between 1990 and 1991 (each not positive).
Xanax, also known as alprazolam, was approved by the FDA in 1981 and belongs to the class of benzodiazepines, a group of depressant medications used to relieve anxiety, the most common mental illness in the US, affecting approximately 40 million adults .
Other common medications in this class include Valium, Klonopin, and Ativan.
Despite the widespread use of Xanax, there are potentially serious side effects.
People taking the drug may experience changes in appetite, nausea, dizziness, drowsiness, changes in sex drive, and irritability.
They may also experience out-of-control actions, loss of control of the legs, numbness in the hands or feet, decreased consciousness, and an unsteady walk.
Additionally, a 2022 study found that anti-anxiety medications can affect a person’s brain cells and neurons, putting them at greater risk of developing cognitive decline later in life.
Dr. Turner, also a professor of psychiatry at Oregon Health & Science University, said SciTech daily: ‘Physicians are well aware of these safety concerns, but there is essentially no doubt about their effectiveness.’
a report from Express Scripts found that about 5 percent of Americans took an anti-anxiety medication in 2019.
However, the use of benzodiazepines for anxiety fell by 12 percent between 2015 and 2019, although prescriptions rose again during the early days of the Covid-19 pandemic when people felt a heightened sense of anxiety.
